Abstract
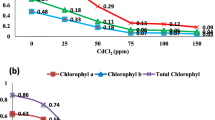
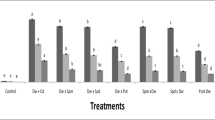
Seeds of pea (Pisum sativum L.) were germinated for 5 days by soaking in distilled water or 5 mM cadmium chloride. Compared to the control, cadmium (Cd) caused a reduction in percent germination and embryo growth. Pyridine nucleotide coenzyme concentrations were determined in cotyledons and embryonic axis. Nicotinamide adenine dinucleotide (NADH) oxidase activity was examined. Cd treatment caused a restriction in levels of reduced coenzyme form in the mitochondria and the post-mitochondrial fraction of cotyledons, and embryonic axis. The oxidized coenzyme form has been accumulated by Cd-treated mitochondria of both tissues. It was also found that NADH oxidase activity was stimulated. The relationship between coenzyme levels, seed germination, pea growth, and Cd stress has been reported.







Similar content being viewed by others
References
Attucci S, Carde JP, Raymond P, Saint-Ges V, Spiteri A, Pradet A (1991) Oxidative phosphorylation by mitochondria extracted from dry sunflower seeds. Plant Physiol 95:390–398
Bansal P, Sharma P, Goyal V (2002) Impact of lead and cadmium on enzyme of citric acid cycle in germinating pea seeds. Biol Plant 45:125–127
Barcelo J, Poschenrieder CH (1990) Plant water relations as affected by heavy metal stress. J Plant Nut 13:1–37
Berger F, Ramirez-Hernandez MH, Ziegler M (2004) The new life of a centenarian: signalling functions of NAD(P). Trends Biochem Sci 29:111–118
Bewley JD, Black M (1994) Seeds: physiology of development and germination, 2nd edn. Plenum, New York
Cannino G, Ferruggia E, Luparello C, Rinaldi AM (2009) Cadmium and mitochondria. Mitochondrion 9:377–384
Chaoui A, Jarrar B, El Ferjani E (2004) Effects of cadmium and copper on peroxidase, NADH oxidase and IAA oxidase activities in cell wall, soluble and microsomal membrane fractions of pea roots. J Plant Physiol 161:1225–1234
Chugh LK, Sawhney SK (1996) Effect of cadmium on germination, amylases and rate of respiration of germinating pea seeds. Environ Pollut 92:1–5
Clijtsters H, Van Assche F (1985) Inhibition of photosynthesis by metals. Photosyn Res 7:31–40
Conley TR, Peng HP, Shih MC (1999) Mutations affecting induction of glycolytic and fermentative genes during germination and environmental stresses in Arabidopsis. Plant Physiol 119:599–607
Douce R, Neuburger M (1989) The uniqueness of plant mitochondria. Annu Rev Plant Physiol Plant Mol Biol 40:371–414
Ernst WHO (1998) Effects of heavy metals in plants at the cellular and organismic level ecotoxicology. In: Gerrit S, Bernd M (eds) Bioaccumulation and biological effects of chemicals, Wiley and Spektrum Akademischer, pp 587–620
Hunt L, Holdsworth MJ, Gray JE (2007) Nicotinamidase activity is important for germination. Plant J 51:341–351
Igamberdiev AU, Gardeström P (2003) Regulation of NAD and NADP-dependent isocitrate dehydrogenases by reduction levels of pyridine nucleotides in mitochondria and cytosol of pea leaves. Biochim Biophys Acta 1606:117–125
Ishida A, Ookubo K, Ono K (1987) Formation of hydrogen peroxide by NAD(P)H oxidation with isolated cell wall-associated peroxidase from cultured liverwort cells, Marchantia polymorpha L. Plant Cell Physiol 28:723–726
Kasimova MR, Grigiene J, Krab K, Hagedorn PH, Flyvbjerg H, Andersen PE, Møller IM (2006) The free NADH concentration is kept constant in plant mitochondria under different metabolic conditions. Plant Cell 18:688–698
Kato-Noguchi H (2002) Hypoxic induction of anoxia tolerance in Avena coleoptiles. J Plant Physiol 159:751–755
Krömer S, Heldt HW (1991) Respiration of pea leaf mitochondria and redox transfer between the mitochondrial and extramitochondrial compartment. Biochim Biophys Acta 1057:42–50
Léon AM, Palma JM, Corpas FJ, Comez M, Romero-Puertas MC, Chatterjee D, Mateos RM, Del Río LA, Sandalio LM (2002) Antioxidative enzymes in cultivars of pepper plants with different sensitivity of cadmium. Plant Physiol Biochem 40:813–820
Logan DC, Millar AH, Sweetlove LJ, Hill SA, Leaver CJ (2001) Mitochondrial biogenesis during germination in maize embryos. Plant Physiol 125:662–672
Mihoub A, Chaoui A, El Ferjani E (2005) Biochemical changes associated with cadmium and copper stress in germinating pea seeds (Pisum sativum L.). C R Biol 328:33–41
Møller IM, Rasmusson AG (1998) The role of NADP in the mitochondrial matrix. Trends Plant Sci 3:21–27
Monerri C, Garcia-Luis A, Guardiola JL (1986) Sugar and starch changes in pea cotyledons during germination. Physiol Plant 67:49–54
Moreland DE, Hussey GG, Shriner CR, Farmer FS (1974) Adenosine phosphates in germinating radish (Raphanus sativus L.) seeds. Plant Physiol 54:560–563
Nawa Y, Asahi T (1971) Rapid development of mitochondria in pea cotyledons during the early stage of germination. Plant Physiol 48:671–674
Noctor G, Queval G, Gakiere B (2006) NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J Exp Bot 57:1603–1620
Obendorf RL, Marcus A (1974) Rapid increase in adenosine 5′-triphosphate during early wheat embryo germination. Plant Physiol 53:779–781
Petit PX, Sommarin M, Pical C, Møller IM (1990) Modulation of endogenous protein phosphorylation in plant mitochondria by respiratory substrates. Physiol Plant 80:493–499
Rahoui S, Chaoui A, El Ferjani E (2008) Differential sensitivity to cadmium in germinating seeds of three cultivars of faba bean (Vicia faba L.). Acta Physiol Plant 30:451–456
Rasmusson AG, Soole KL, Elthon TE (2004) Alternative NAD(P)H dehydrogenases of plant mitochondria. Annu Rev Plant Biol 55:23–39
Raymond P, Al-Ani A, Pradet A (1985) ATP production by respiration and fermentation, and energy charge during aerobis and anaerobis in twelve fatty and starchy seeds. Plant Physiol 79:879–884
Rodríguez-Serrano M, Romero-Puertas MC, Pazmiño DM, Testillano PS, Risueño MC, Del Río LA, Sandalio LM (2009) Cellular response of pea plants to cadmium toxicity: cross-talk between reactive oxygen species, nitric oxide, and calcium. Plant Physiol 150:229–243
Saher S, Fernandez-Garcia N, Piqueras A, Hellin E, Olmos E (2005) Reducing properties, energy efficiency and carbohydrate metabolism in hyperhydric and normal carnation shoots cultured in vitro: a hypoxia stress? Plant Physiol Biochem 43:573–582
Sanita di Toppi L, Gabbreilli R (1999) Response to cadmium in higher plants. Environ Exp Bot 41:105–130
Schwarzländer M, Fricker MD, Sweetlove LJ (2009) Monitoring the in vivo redox state of plant mitochondria: effect of respiratory inhibitors, abiotic stress and assessment of recovery from oxidative challenge. Bioch Biophys Acta 1787:468–475
Shetty PJ, Atallah MT, Shetty K (2002) Effect of UV treatment on proline-linked pentose phosphate pathway for phenolic and L-DOPA synthesis in dark germinated Vicia faba. Process Biochem 37:1285–1295
Smiri M, Chaoui A, El Ferjani E (2009) Respiratory metabolism in the embryonic axis of germinating pea seed exposed to cadmium. J Plant Physiol 166:259–269
Smiri M, Chaoui A, Rouhier N, Jacquot JP, El Ferjani E (2010) Effect of cadmium on resumption of respiration in cotyledons of germinating pea seeds. Ecotox Environ Saf. doi:10.1016/j.ecoenv.2010.05.015
Stupnikova I, Benamar A, Tolleter D, Grelet J, Borovskii G, Dorne A-J, Macherel D (2006) Pea seed mitochondria are endowed with a remarkable tolerance to extreme physiological temperatures. Plant Physiol 140:326–335
Tommasi F, Paciolla C, De Pinto MC, De Gara L (2001) A comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. J Exp Bot 52:1647–1654
Van Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Wagner GJ (1993) Accumulation of cadmium in crop plants and its consequences to human health. Adv Agron 51:173–212
Woolhouse HW (1983) Toxicity and tolerance in the responses of plants to metals. In: Lange OL, Nobel PS, Osmond CB, Ziegler H (eds) Encyclopaedia of plant physiology. Springer, Berlin, pp 245–300
Zhao Z, Hu X, Ross CW (1987) Comparison of tissue preparation methods for assay of nicotinamide coenzymes. Plant Physiol 84:987–988
Acknowledgements
We are thankful to three anonymous reviewers for helpful comments on the manuscript. Financial support for this work was received from the Tunisian Ministry of Higher Education, Scientific Research, and Technology and INRA-Henri Poincaré University (France).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smiri, M., Chaoui, A., Rouhier, N. et al. NAD pattern and NADH oxidase activity in pea (Pisum sativum L.) under cadmium toxicity. Physiol Mol Biol Plants 16, 305–315 (2010). https://doi.org/10.1007/s12298-010-0033-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12298-010-0033-7