Abstract
Pulmonary arterial hypertension (PAH) is a lethal, progressive disease with a complex pathogenesis. Bosentan, a dual endothelin receptor antagonist, and sildenafil, a phosphodiesterase type 5 inhibitor, are used to treat PAH. In this study, we aimed to develop a liquid chromatography-tandem mass spectrometry method (LC–MS/MS) to measure the levels of bosentan, sildenafil, and their active metabolites in patients with PAH. We have developed an LC–MS/MS measurement procedure using a liquid–liquid extraction to measure serum drug concentrations and validated the procedure according to Clinical and Laboratory Standards Institute (CLSI) protocols.
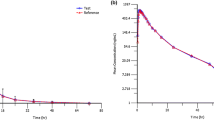
Finally, the validated method was used to measure the levels of sildenafil, bosentan, and their metabolite in pediatric PAH patients. The method was linear in the range of 0.975–1000 ng/ml and 0.76–3125 ng/ml for sildenafil and bosentan, respectively. LOQ values of sildenafil and bosentan were determined as 1.95 and 1.50 ng/ml, respectively. A method for measuring the levels of sildenafil and bosentan was developed that is rapid, robust, inexpensive, and requires a small serum volume. In addition, the validated method measured these drugs’ levels and metabolites in pediatric patients with PAH. The results show that the established method can routinely monitor drug levels.

Similar content being viewed by others
References
Memon HA, Park MH. Pulmonary arterial hypertension in women. Methodist Debakey Cardiovasc J. 2017;13(4):224–37. https://doi.org/10.14797/mdcj-13-4-224.
Chambers E, Wagrowski-Diehl DM, Lu Z, Mazzeo JR. Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J Chromatogr B. 2007;852(1–2):22–34. https://doi.org/10.1016/j.jchromb.2006.12.030.
Besinque LP. The myth of the stable pulmonary arterial hypertension patient. Am J Manag Care. 2019;25(3):47–52.
Sitbon O, Morrell N. Pathways in pulmonary arterial hypertension: the future is here. Eur Respir Rev. 2012;21(126):321–7. https://doi.org/10.1183/09059180.00004812.
Limoncella S, Lazzaretti C, Paradiso E, D’Alessandro S, Barbagallo F, Pacifico S, et al. Phosphodiesterase (PDE) 5 inhibitors sildenafil, tadalafil and vardenafil impact cAMP-specific PDE8 isoforms-linked second messengers and steroid production in a mouse Leydig tumor cell line. Mol Cell Endocrinol. 2022;542: 111527. https://doi.org/10.1016/j.mce.2021.111527.
Jain MS, Koradia SK. Phosphodiesterase-5 (PDE 5) inhibitors in the treatment of erectile dysfunction: a review. Asian J Pharm Res. 2023;13(1):63–7. https://doi.org/10.52711/2231-5691.2023.00012.
Kuno Y, Iyoda M, Shibata T, Hirai Y, Akizawa T. Sildenafil, a phosphodiesterase type 5 inhibitor, attenuates diabetic nephropathy in non-insulin-dependent Otsuka Long–Evans Tokushima Fatty rats. Br J Pharmacol. 2011;162(6):1389–400. https://doi.org/10.1111/j.1476-5381.2010.01149.x.
Yan H, Yu W. Retinal toxicity of long term overdose of sildenafil citrate: a case report. Am J Ophthalmol Case Rep. 2023;29: 101761. https://doi.org/10.1016/j.ajoc.2022.101761.
Sheweita SA, Alian DME, Haroun M, Nounou MI, Patel A, El-Khordagui L. Chitosan nanoparticles alleviated the adverse effects of sildenafil on the oxidative stress markers and antioxidant enzyme activities in rats. Oxid Med Cell Longev. 2023. https://doi.org/10.1155/2023/9944985.
Tang P-F, Zheng X, Hu X-X, Yang C-C, Chen Z, Qian J-C, et al. Functional measurement of CYP2C9 and CYP3A4 allelic polymorphism on sildenafil metabolism. Drug Des Develop Ther. 2020. https://doi.org/10.2147/DDDT.S268796.
Kuntz M, Leiva-Juarez MM, Luthra S. Systematic review of randomized controlled trials of endothelin receptor antagonists for pulmonary arterial hypertension. Lung. 2016;194:723–32. https://doi.org/10.1007/s00408-016-9928-6.
Roberts KE, Preston IR. Safety and tolerability of bosentan in the management of pulmonary arterial hypertension. Drug Des Develop Ther. 2009. https://doi.org/10.2147/DDDT.S3786.
Dingemanse J, van Giersbergen PLJC. Clinical pharmacology of bosentan, a dual endothelin receptor antagonist. Clin Pharm. 2004;43(15):1089–115.
Dingemanse J, Bodin F, Weidekamm E, Kutz K, van Giersbergen P. Influence of food intake and formulation on the pharmacokinetics and metabolism of bosentan, a dual endothelin receptor antagonist. J Clin Pharmacol. 2002;42(3):283–9. https://doi.org/10.1177/00912700222011300.
Weber C, Gasser R, Hopfgartner GJDM. Disposition Absorption, excretion, and metabolism of the endothelin receptor antagonist bosentan in healthy male subjects. Drug Metab Dispos. 1999;27(7):810–5.
Ausó E, Gómez-Vicente V, Esquiva G. Visual side effects linked to sildenafil consumption: an update. Biomedicines. 2021;9(3):291. https://doi.org/10.3390/biomedicines9030291.
Fagelman E, Fagelman A, Shabsigh R. Efficacy, safety, and use of sildenafil in urologic practice. Urology. 2001;57(6):1141–4. https://doi.org/10.1016/S0090-4295(01)00984-0.
O’Callaghan D, Gaine SP. Bosentan: a novel agent for the treatment of pulmonary arterial hypertension. Int J Clin Pract. 2004;58(1):69–73. https://doi.org/10.1111/j.1368-5031.2004.0098.x.
Siehr SL, McCarthy EK, Ogawa MT, Feinstein JA. Reported sildenafil side effects in pediatric pulmonary hypertension patients. Front Pediatr. 2015;3:12. https://doi.org/10.3389/fped.2015.00012.
Fu W, Chen P, Xia J, Fu L, Shen Y, He W, et al. Efficacy and safety of endothelin receptor antagonists combined with phosphodiesterase 5 inhibitor in the treatment of pulmonary arterial hypertension: a network meta-analysis. Chin J Tuber Resp Dis. 2022;45(2):158–70. https://doi.org/10.3760/cma.j.cn112147-20210707-00473.
Maneenil G, Talek S, Thatrimontrichai A, Janjindamai W, Dissaneevate S. The use of bosentan and sildenafil as rescue therapy in persistent pulmonary hypertension of the newborn: a single center’s experience. Prog Pediatr Cardiol. 2022;67: 101575. https://doi.org/10.1016/j.ppedcard.2022.101575.
Wang R, Wei M, Wang J, Huang X, Yan Q, Wang S, et al. A network meta-analysis of the efficacy and safety of targeted drug combinations in the treatment of pulmonary arterial hypertension. LWW. 2023. https://doi.org/10.1097/CD9.0000000000000105.
Eerkes A, Addison T, Naidong W. Simultaneous assay of sildenafil and desmethylsildenafil in human plasma using liquid chromatography–tandem mass spectrometry on silica column with aqueous–organic mobile phase. J Chromatogr B. 2002;768(2):277–84. https://doi.org/10.1016/S1570-0232(01)00602-X.
Lausecker B, Hopfgartner G. Determination of an endothelin receptor antagonist in human plasma by narrow-bore liquid chromatography and ionspray tandem mass spectrometry. J Chromatogr A. 1995;712(1):75–83. https://doi.org/10.1016/0021-9673(95)00331-G.
Tanaka S, Uchida S, Hakamata A, Miyakawa S, Odagiri K, Inui N, et al. Simultaneous LC-MS analysis of plasma concentrations of sildenafil, tadalafil, bosentan, ambrisentan, and macitentan in patients with pulmonary arterial hypertension. Die Pharm Int J Pharm Sci. 2020;75(6):236–9. https://doi.org/10.1691/ph.2020.0021.
Rashid J, Ahsan F. A highly sensitive LC–MS/MS method for concurrent determination of sildenafil and rosiglitazone in rat plasma. J Pharm Biomed Anal. 2016;129:21–7. https://doi.org/10.1016/j.jpba.2016.06.022.
Qiu X, Zhao J, Wang Z, Xu Z, Xu R. Simultaneous determination of bosentan and glimepiride in human plasma by ultra performance liquid chromatography tandem mass spectrometry and its application to a pharmacokinetic study. J Pharm Biomed Anal. 2014;95:207–12. https://doi.org/10.1016/j.jpba.2014.03.011.
Liew KB, Loh GOK, Tan YTF, Peh KK. Simultaneous quantification of sildenafil and N-desmethyl sildenafil in human plasma by UFLC coupled with ESI-MS/MS and pharmacokinetic and bioequivalence studies in Malay population. Biomed Chromatogr. 2015;29(6):953–60. https://doi.org/10.1002/bmc.3378.
Challa BR, Awen BZ, Chandu BR, Khagga M, Bannoth CK, Kanala K, et al. Sildenafil and N-desmethyl sildenafil quantification in human plasma by HPLC coupled with ESI-MS/MS detection: application to bioequivalence study. Anal Methods. 2010;2(8):1043–50. https://doi.org/10.1039/C0AY00062K.
Yokoyama Y, Tomatsuri M, Hayashi H, Hirai K, Ono Y, Yamada Y, et al. Simultaneous microdetermination of bosentan, ambrisentan, sildenafil, and tadalafil in plasma using liquid chromatography/tandem mass spectrometry for pediatric patients with pulmonary arterial hypertension. J Pharm Biomed Anal. 2014;89:227–32. https://doi.org/10.1016/j.jpba.2013.11.007.
Acknowledgements
This research was supported by Selcuk University Scientific Research Projects Coordination (Project Number 17202067). The authors would like to thank Selcuk University for funding this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest relevant to the content of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bik, M.A., Onmaz, D.E., Gharab, K.M.K. et al. Development of a Robust, Rapid and Reliable Tandem Mass Spectrometry Method for the Measurement of Sildenafil, Bosentan and their Major Metabolites. Ind J Clin Biochem (2024). https://doi.org/10.1007/s12291-024-01215-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12291-024-01215-x