Abstract
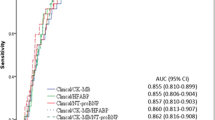
Acute coronary syndrome (ACS) is caused by decreased blood flow to the heart muscle after plaque rupture and thrombus formation, leading to ischemia and infarction. Cofilins are the proteins involved in actin dynamics by severing and dissociating actin filaments. Abnormal cofilins are associated with myopathies, idiopathic dilated cardiomyopathies, etc. As ACS prevalence is increasing and there is a need to find specific biomarkers for ACS diagnosis, the study aimed to assess the diagnostic utility of serum cofilin-1 (CFL-1) and 2 (CFL-2) levels in ACS patients. Forty-five ACS patients as cases and 45 healthy participants as controls were recruited in a case–control pilot study. Collected blood was used to measure serum CFL-1, CFL-2 and heart-type fatty acid-binding protein (H-FABP, a marker of myocardial infarction) levels. Serum CFL-2 levels were significantly lower in cases compared to controls (2.93 [1.95–3.32] vs. 4.35 [3.4–6.61], p < 0.05). No significant difference was observed in serum CFL-1 levels between groups. Serum CFL-2 levels were negatively associated with creatine kinase–total, creatine kinase–MB, creatine kinase–MB relative index, troponin-T, and H-FABP (p < 0.05). Binary logistic regression showed lower CFL-2 levels were associated with a 2.45-fold increased ACS risk. ROC analysis showed no advantage of serum CFL-2 levels over serum H-FABP levels for ACS diagnosis (AUC: 0.120 Vs 0.710, p > 0.05). In conclusion, serum CFL-1 and 2 levels may not have greater significance in ACS diagnosis than traditional cardiac biomarkers. However, further cohort studies with a larger sample size must confirm the study’s findings.

Similar content being viewed by others
References
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. https://doi.org/10.1016/j.jacc.2020.11.010.
Deora S, Kumar T, Ramalingam R, Nanjappa MC. Demographic and angiographic profile in premature cases of acute coronary syndrome: analysis of 820 young patients from South India. Cardiovasc Diagn Ther. 2016;6(3):193–8. https://doi.org/10.21037/cdt.2016.03.05.
Bhatt DL, Lopes RD, Harrington RA. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327(7):662–75. https://doi.org/10.1001/jama.2022.0358.
Wang XY, Zhang F, Zhang C, Zheng LR, Yang J. The biomarkers for acute myocardial infarction and heart failure. Biomed Res Int. 2020;2020:2018035. https://doi.org/10.1155/2020/2018035.
Abe T, Samuel I, Eferoro E, Samuel AO, Monday IT, Olunu E, et al. The diagnostic challenges associated with type 2 myocardial infarction. Int J Appl Basic Med Res. 2021;11(3):131–8. https://doi.org/10.4103/ijabmr.IJABMR_210_20.
Xu S, Jiang J, Zhang Y, Chen T, Zhu M, Fang C, et al. Discovery of potential plasma protein biomarkers for acute myocardial infarction via proteomics. J Thorac Dis. 2019;11(9):3962–72. https://doi.org/10.21037/jtd.2019.08.100.
Wu Y, Pan N, An Y, Xu M, Tan L, Zhang L. Diagnostic and prognostic biomarkers for myocardial infarction. Front Cardiovasc Med. 2021;7: 617277. https://doi.org/10.3389/fcvm.2020.617277.
Tanaka K, Takeda S, Mitsuoka K, Oda T, Kimura-Sakiyama C, Maéda Y, et al. Structural basis for cofilin binding and actin filament disassembly. Nat Commun. 2018;9(1):1860. https://doi.org/10.1038/s41467-018-04290-w.
Maciver SK, Hussey PJ. The ADF/cofilin family: actin-remodeling proteins. Genome Biol. 2002;3(5):3007.
Ehler E. Actin-associated proteins and cardiomyopathy-the “unknown” beyond troponin and tropomyosin. Biophys Rev. 2018;10(4):1121–8. https://doi.org/10.1007/s12551-018-0428-1.
Kanellos G, Zhou J, Patel H, Radgway RA, Huels D, Gurniak CB, et al. ADF and cofilin1 control actin stress fibers, nuclear integrity, and cell survival. Cell Rep. 2015;13(9):1949–64. https://doi.org/10.1016/j.celrep.2015.10.056.
Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actine dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94(1):235–63. https://doi.org/10.1152/physrev.00018.2013.
Kremneva E, Makkonen MH, Skwarek-Maruszewska A, Gateva G, Michelot A, Dominguez R, et al. Cofilin-2 controls actin filament length in muscle sarcomeres. Dev cell. 2014;31(2):215–26. https://doi.org/10.1016/j.devcel.2014.09.002.
Mohri K, Suzuki-Toyota F, Obinata T, Sato N. Chimeric mice with deletion of Cfl2 that encodes muscle-type cofilin (MCF or Cofilin-2) results in defects of striated muscles, both skeletal and cardiac muscles. Zoolog Sci. 2019;36(2):112–9. https://doi.org/10.2108/zs180151.
Subramanian K, Gianni D, Balla C, Assenza GE, Joshi M, Semigran MJ, et al. Cofilin-2 phosphorylation and sequestration in myocardial aggregates: novel pathogenetic mechanisms for idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2015;65(12):1199–214. https://doi.org/10.1016/j.jacc.2015.01.031.
Chatzifrangkeskou M, Yadin D, Marais T, Chardonnet S, Cohen-Tannoudji M, Mougenot N, et al. Cofilin-1 phosphorylation catalyzed by ERK1/2 alters cardiac actin dynamics in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum Mol Genet. 2018;27(17):3060–78. https://doi.org/10.1093/hmg/ddy215.
Nguyen K, Chau VQ, Mauro AG, Durrant D, Toldo S, Abbate A, et al. Hydrogen sulfide therapy suppresses cofilin-2 and attenuates ischemic heart failure in a mouse model of myocardial infarction. J Cardiovasc Pharmacol Ther. 2020;25(5):472–83. https://doi.org/10.1177/1074248420923542.
Wang L, Buckley AF, Spurney RF. Regulation of cofilin phosphorylation in glomerular podocytes by testis specific kinase 1 (TESK1). Sci Rep. 2018;8(1):12286. https://doi.org/10.1038/s41598-018-30115-3.
Bamburg JR, Minamide LS, Wiggan O, Tahtamouni LH, Kuhn TB. Cofilin and actin dynamics: multiple modes of regulation and their impacts in neuronal development and degeneration. Cells. 2021;10(10):2726. https://doi.org/10.3390/cells10102726.
Sun Y, Liang L, Dong M, Li C, Liu Z, Gao H. Cofilin 2 in serum as a novel biomarker for Alzheimer’s Disease in Han Chinese. Front Aging Neurosci. 2019;11:214. https://doi.org/10.3389/fnagi.2019.00214.
Xu J, Huang Y, Zhao J, Wu L, Qi Q, Liu Y, et al. Cofilin: a promising protein implicated in cancer metastasis and apoptosis. Front Cell Dev Biol. 2021;9: 599065. https://doi.org/10.3389/fcell.2021.599065.
Backus BE, Six AJ, Kelder JH, Gibler WB, Moll FL, Doevendans PA. Risk scores for patients with chest pain: evaluation in the emergency department. Curr Cardiol Rev. 2011;7(1):2–8. https://doi.org/10.2174/157340311795677662.
Liu N, Ng JC, Ting CE, Sakamoto JT, Ho AF, Koh ZX, et al. Clinical scores for risk stratification of chest pain patients in the emergency department: an updated systemic review. J Emerg Crit Care Med. 2018;2:16. https://doi.org/10.21037/jeccm.2018.01.10.
Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013;4(2):627–35.
Baumann AAW, Mishra A, Worthley MI, Nelson AJ, Psaltis PJ. Management of multivessel coronary artery disease in patients with non-ST-elevation myocardial infarction: a complex path to precision medicine. Ther Adv Chronic Dis. 2020;11:2040622320938527. https://doi.org/10.1177/2040622320938527.
Baumann AA, Tavella R, Air TM, Mishra A, Montarello NJ, Arstall M, et al. Prevalence and real-world management of NSTEMI with multivessel disease. Cardiovasc Diagn Ther. 2022;12(1):1–11. https://doi.org/10.21037/cdt-21-518.
Sharma YP, Santosh Vemuri K, Bootla D, Kanabar K, Pruthvi CR, Kaur N, et al. Epidemiological profile, management and outcomes of patients with acute coronary syndrome: single centre experience from a tertiary care hospital in North India. Indian Heart J. 2021;73(2):174–9. https://doi.org/10.1016/j.ihj.2020.11.149.
Wang D, Enck J, Howell BW, Olson EC. Ethanol exposure transiently elevates but persistently inhibits tyrosine kinase activity and impairs the growth of the nascent apical dendrite. Mol Neurobiol. 2019;56(8):5749–62. https://doi.org/10.1007/s12035-019-1473-x.
Limatola N, Vasilev F, Santella L, Chun JT. Nicotine induces polyspermy in sea urchin eggs through a non-cholinergic pathway modulating actin dynamics. Cells. 2019;9(1):63. https://doi.org/10.3390/cells9010063.
Babes EE, Bustea C, Behl T, Abdel-Daim MM, Nechifor AC, Stoicescu M, et al. Acute coronary syndromes in diabetic patients, outcome, revascularization, and antithrombotic therapy. Biomed Pharmacother. 2022;148: 112772. https://doi.org/10.1016/j.biopha.2022.112772.
Dhungana SP, Mahato AK, Ghimire R, Shreewastav RK. Prevalence of dyslipidemia in patients with acute coronary syndrome admitted at tertiary care hospital in Nepal: A descriptive cross-sectional study. JNMA J Nepal Med Assoc. 2020;58(224):204–208. https://doi.org/10.31729/jnma.4765.
Hien TT, Turczyńska KM, Dahan D, Ekman M, Grossi M, Sjögren J, et al. Elevated glucose levels promote contractile and cytoskeletal gene expression in vascular smooth muscle via Rho/Protein kinase C and actin polymerization. J Biol Chem. 2016;291(7):3552–68. https://doi.org/10.1074/jbc.M115.654384.
Hansson B, Morén B, Fryklund C, Vliex L, Wasserstrom S, Albinsson S, et al. Adipose cell size changes are associated with a drastic actin remodeling. Sci Rep. 2019;9:12941. https://doi.org/10.1038/s41598-019-49418-0.
Sumi T, Matsumoto K, Takai Y, Nakamura T. Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J Cell Biol. 1999;147(7):1519–32. https://doi.org/10.1083/jcb.147.7.1519.
Gerbino A, Procino G, Svelto M, Carmosino M. Role of lamin A/C gene mutations in the signaling defects leading to cardiomyopathies. Front Physiol. 2018;9:1356. https://doi.org/10.3389/fphys.2018.01356.
Kueh HY, Charras GT, Mitchison TJ, Brieher WM. Actin disassembly by cofilin, coronin, and Aip1 occurs in bursts and is inhibited by barbed-end cappers. J Cell Biol. 2008;182(2):341–53. https://doi.org/10.1083/jcb.200801027.
Yang FH, Pyle WG. Reduced cardiac CapZ protein protects hearts against acute ischemia-reperfusion injury and enhances preconditioning. J Mol Cell Cardiol. 2012;52(3):761–72.
Özdemİr A, Ark M. A novel ROCK inhibitor: off-target effects of metformin. Turk J Biol. 2021;45(1):35–45. https://doi.org/10.3906/biy-2004-12.
Funding
This work was supported by an Intramural research grant from JIPMER, Puducherry, India (No: JIP/Res/Intramural/Phs 1/2021-22 dated May 20, 2021).
Author information
Authors and Affiliations
Contributions
A.K.G. obtained patient data and blood samples, analyzed blood samples and data; P.S.A. designed the study, analyzed the data and contributed to the writing of the manuscript; K.V.V designed the study and contributed for the selection of study participants. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report any conflict of interest.
Ethics Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Approval of the Institute Human Ethics Committee was taken (JIP/IEC/2021/014 Dated 03/03/2021). Written informed consent was taken from the study participants before recruitment and after informing them that the study was not part of their treatment.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ganguli, A.K., Adole, P.S. & Vinod, K.V. Exploring the Diagnostic Utility of Serum Cofilin-1 and 2 Levels in Patients with Acute Coronary Syndrome: A Case–Control Pilot Study. Ind J Clin Biochem (2024). https://doi.org/10.1007/s12291-024-01195-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12291-024-01195-y