Abstract
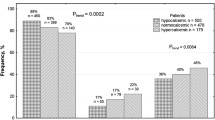
Murine studies stipulate Peptidyl-prolyl cis-trans isomerase NIMA-interacting 1 (PIN-1) as a key mediator of PTH mRNA stability and is acknowledged by genome-wide-association-studies as secondary hyperparathyroidism defining trait in chronic kidney disease. Therefore, we hypothesize that molecular variants of the PIN-1 gene might affect the incidence and predisposition to secondary hyperparathyroidism in chronic renal insufficiency. A total of 281 adult participants, including 124 chronic kidney disease patients with secondary hyperparathyroidism and 157 healthy controls, were genotyped using polymerase chain reaction-restriction fragment length polymorphism method to unravel the effects of genetic risk loci (PIN-1 gene; − 667C > T; rs2233679) on the susceptibility to secondary hyperparathyroidism in chronic kidney disease. Suitable descriptive statistics was used for different variables. The genotype (x2: 8.03, 2 d. f. p = 0.0181) and allele distribution (x2: 7.27, 2 d. f. p: 0.007) of the − 667C > T variant differed significantly in cases and controls, with no deviation from Hardy–Weinberg equilibrium in either affected or control group. The observed frequencies of T allele of PIN-1 − 667 C > T SNP was significantly high in CKD-SHPT group compared to control group (52.41% vs. 41.71%; p: 0.0118). TT variant of PIN-1 − 667C > T SNP was associated with increased risk for occurrence of CKD-SHPT in univariate analysis (OR: 4.6, p: 0.0032, 95% CI: 1.66–12.76). Further in multivariate analysis, both TT (OR: 3.84, p: 0.002, 95% CI: 0.79–9.26) and CT + TT (OR: 2.51, p: 0.031, 95% CI: 0.64–8.68) variants were independently associated with increased risk for CKD-SHPT, emphasizing the deleterious effect of minor T allele. Serum PTH, phosphorus levels were significantly high in CT and TT genotypes compared to CC genotype of PIN-1 − 667C > T SNP (p = 0.001). PIN-1 promoter functional SNP (− 667C > T; rs2233679) appeared to be an important genetic determinant in etiopathogenesis of CKD-SHPT and genetic variants of this SNP influences the risk stratification and might serve as a predictive marker. Thus, rs2233679 can be useful for outcome predictions during diagnostic processes.

Similar content being viewed by others
References
K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease. Evaluation, classification and stratification. Am J Kidney Dis. 2002;39:S1-266.
KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for Anemia in Chronic Kidney Disease KDOQI; National Kidney Foundation. Am J Kidney Dis 2006;47:S11–145.
Zhao Y, Zhang LL, Ding FX, Cao P, Qi YY, Wang J. Pin1 and secondary hyperparathyroidism of chronic kidney disease: gene polymorphisms and protein levels. Ren Fail. 2017;39(1):159–65.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;76(S113):S1-130.
Nechama M, Uchida T, Mor Yosef-Levi I, Silver J, Naveh-Many T. The peptidyl-prolyl isomerase Pin1 determines parathyroid hormone mRNA levels and stability in rat models of secondary hyperparathyroidism. J Clin Invest. 2009;119(10):3102–14.
Kilav-Levin R, Hassan A, Nechama M, Shilo V, Silver J, Ben-Dov IZ, et al. Post-transcriptional mechanisms regulating parathyroid hormone gene expression in secondary hyperparathyroidism. FEBS J. 2020;287(14):2903–13.
Galat A. Peptidylprolyl cis/trans isomerases (immunophilins): Biological diversity-targets-functions. Curr Top Med Chem. 2003;3:1315–47.
Chen D, Wang L, Lee TH. Post-translational modifications of the peptidyl-prolyl isomerase Pin1. Front Cell Dev Biol. 2020;8:129.
Fila C, Metz C, Van Der Sluijs P. Juglone inactivates cysteine-rich proteins required for progression through mitosis. J Biol Chem. 2008;283:21714–24.
Lu J, Hu Z, Wei S, Wang LE, Liu Z, El-Naggar AK, et al. A novel functional variant (− 842G > C) in the PIN1 promoter contributes to decreased risk of squamous cell carcinoma of the head and neck by diminishing the promoter activity. Carcinogenesis. 2009;3010:1717–21.
Wang L, Zhou Y, Chen D, Lee TH. Peptidyl-prolyl cis/trans isomerase pin1 and Alzheimer’s disease. Front Cell Dev Biol. 2020;8:355.
Jeanpierre M. A rapid method for the purification of DNA from blood. Nucleic Acids Res. 1987;15(22):9611.
Tataurov AV, You Y, Owczarzy R. Predicting ultraviolet spectrum of single stranded and double stranded deoxyribonucleic acids. Biophys Chem. 2008;133:66–70.
Cortadellas O, Fernández del Palacio MJ, Talavera J, Bayón A. Calcium and phosphorus homeostasis in dogs with spontaneous chronic kidney disease at different stages of severity. J Vet Intern Med. 2010;24(1):73–9.
Hu X, Chen LF. Pinning down the transcription: a role for peptidyl-prolyl cis-trans isomerase Pin1 in gene expression. Front Cell Dev Biol. 2020;8:179.
Arora K, Goyal G, Soin D, Kumar S, Arora H, Garg C. Correlation of parathyroid hormone levels with mineral status in end-stage renal disease patients. Indian J Endocr Metab. 2018;22:735–9.
Tomasello S, Pharm D. Secondary hyperparathyroidism and chronic kidney disease. Diabetes Spectr. 2008;21:19–25.
Holick MF. Vitamin D for health and in chronic kidney disease. Sem Dial. 2005;18:266–75.
Huang L, Mo Z, Li S, Qin X. The association between PIN1 genetic polymorphisms and the risk of chronic hepatitis B and hepatitis B virus-related liver cirrhosis: a case-control study. Med (Baltimore). 2018;97(35):e12123.
Yao J, Wang JM, Wang ZL, Wu YN. The Pin1 gene polymorphism and the susceptibility of oral squamous cell carcinoma in East China. Cancer Biomark. 2014;14(6):441–7.
Han CH, Lu J, Wei Q, Bondly ML, Brewster AM, Yu TK, et al. The functional promoter polymorphism (− 842G > C) in the PIN1 gene is associated with decreased risk of breast cancer in non-Hispanic white women 55 years and younger. Breast Cancer Res Treat. 2010;1229(1):243–9.
Naidu R, Har YC, Taib NA. Analysis of peptidyl-propyl-cis/trans isomerase 1 (PIN1) gene − 842(G > C) and − 667(T > C) polymorphic variants in relation to breast cancer risk and clinico-pathological parameters. Scand J Clin Lab Invest. 2011;71:500–6.
Arosio B, Segat L, Milanese M, Galimberti L, Calabresi C, Zanetti M, et al. PIN-1 promoter polymorphisms in mild cognitive impairment and susceptibility to Alzheimer’s disease: a preliminary report. Aging Clin Exp Res. 2007;19(5):406–9.
Levi R, Ben-Dov IZ, Lavi-Moshayoff V, Dinur M, Martin D, Naveh-Many T, et al. Increased parathyroid hormone gene expression in secondary hyperparathyroidism of experimental uremia is reversed by calcimimetics: correlation with posttranslational modification of the trans acting factor AUF1. J Am Soc Nephrol. 2006;17(1):107–12.
Naveh-Many T, Volovelsky O. Parathyroid cell proliferation in secondary hyperparathyroidism of chronic kidney disease. Int J Mol Sci. 2020;21(12):4332.
Kilav R, Silver J, Naveh-Many T. A conserved cis-acting element in the parathyroid hormone 3′-untranslated region is sufficient for regulation of RNA stability by calcium and phosphate. J Biol Chem. 2001;276:8727–33.
Kilav R, Bell O, Le SY, Silver J, NavehMany T. The parathyroid hormone mRNA 3′-untranslated region AU-rich element is an unstructured functional element. J Biol Chem. 2004;279:2109–16.
Nechama M, Ben-Dov IZ, Briata P, Gherzi R, Naveh-Many T. The mRNA decay promoting factor K-homology splicing regulator protein post-transcriptionally determines parathyroid hormone mRNA levels. FASEB J. 2008;22:3458–68.
Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, et al. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107(4):451–64.
Author information
Authors and Affiliations
Contributions
On behalf of all the contributors I will act as guarantor and will correspond with the journal from this point onward.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, D.D., Parchwani, D., Vachhani, U. et al. A Molecular Insight of the Role of PIN-1 Promoter Polymorphism (− 667C > T; rs2233679) in Chronic Kidney Disease Patients with Secondary Hyperparathyroidism. Ind J Clin Biochem 37, 319–327 (2022). https://doi.org/10.1007/s12291-021-00997-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-021-00997-8