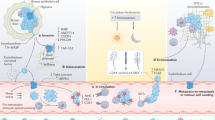
Abstract
Circulating tumour cells (CTCs), are disseminated tumour cells found in the blood in solid tumour malignancies. Identification of CTCs act as emerging tools in the field of the Liquid Biopsy. Majority of the studies focused on detection and enumeration of CTCs due to technological challenges those results from the rarity of CTCs in the blood. Enumeration of CTCs has already proven their value as prognostic as well as predictive biomarkers for disease prognosis. However, recent advances in technology permitted to study the molecular and functional features of CTCs and these features have the potential to change the diagnostic, prognostic and predictive landscape in oncology. In this review, we summarize the paradigm shift in the field of liquid biopsy-based cancer diagnostics using CTC isolation and detection. We have discussed recent advances in the technologies for molecular characterization of CTCs which have aided a shift from CTC enumeration to an in-depth analysis of the CTC genome, transcriptomes, proteins, epigenomes along with various functional features. Finally, as a prognosticating strategy, the potentials of CTCs as a tool of liquid biopsy to predict micrometastasis, monitor prognosis and how to use them as an additional tool for cancer staging has been discussed.




Similar content being viewed by others
References
Pantel K, Alix-Panabieres C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16:398–406.
Batth IS, Mitra A, Manier S, et al. Circulating tumor markers: harmonizing the yin and yang of CTCs and ctDNA for precision medicine. Ann Oncol. 2017;28(3):468–77.
Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–48.
Wan JCM, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–38.
Heitzer E, Ulz P, Geigl JB. Circulating tumor DNA as a liquid biopsy for cancer. Clin Chem. 2015;61:112–23.
Alix-Panabieres C, Pantel K. Challenges in circulating tumour cell research. Nat Rev Cancer. 2014;14:623–31.
Alix-Panabieres C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor dna as liquid biopsy. Cancer Discov. 2016;6:479–91.
Bardelli A, Pantel K. Liquid biopsies, what we do not know (yet). Cancer Cell. 2017;31:172–9.
Amorim MG, et al. A total transcriptome profiling method for plasma-derived extracellular vesicles: applications for liquid biopsies. Sci Rep. 2017;7:14395.
Chan KC, et al. Noninvasive detection of cancer associated genome-wide hypomethylation and copy number aberrations by plasma DNA bisulfite sequencing. Proc Natl Acad Sci USA. 2013;110:18761–8.
Kim Y, et al. Targeted proteomics identifies liquid biopsy signatures for extracapsular prostate cancer. Nat Commun. 2016;7:11906.
Mayers JR, et al. Elevation of circulating branched chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat Med. 2014;20:1193–8.
Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumour cells: a multifunctional biomarker. Clin Cancer Res. 2014;20:2553–68.
Alix-Panabieres C, Mader S, Pantel K. Epithelial mesenchymal plasticity in circulating tumor cells. J Mol Med. 2017;95:133–42.
Ohnaga T, Takei Y, Nagata T, Shimada Y. Highly efficient capture of cancer cells expressing EGFR by microfluidic methods based on antigen-antibody association. Sci Rep. 2018;8:12005.
Santana SM, Liu H, Bander NH, Gleghorn JP, Kirby BJ. Immunocapture of prostate cancer cells by use of anti-PSMA antibodies in microdevices. Biomed Microdevices. 2012;14:401–7.
Coumans FA, van Dalum G, Beck M, Terstappen LW. Filtration parameters influencing circulating tumor cell enrichment from whole blood. PLoS ONE. 2013;8:e61774.
Sun N, Li X, Wang Z, Li Y, Pei R. High-purity capture of CTCs based on micro-beads enhanced isolation by size of epithelial tumor cells (ISET) method. Biosens Bioelectron. 2018;102:157–63.
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904.
Bhagwat N, et al. An integrated flow cytometry-based platform for isolation and molecular characterization of circulating tumor single cells and clusters. Sci Rep. 2018;8:5035.
Lopresti A, Malergue F, Bertucci F, Liberatoscioli ML, Garnier S, DaCosta Q, et al. Sensitive and easy screening for circulating tumor cells by flow cytometry. JCI Insight. 2019. https://doi.org/10.1172/jci.insight.128180.
Soler A, Cayrefourcq L, Mazel M, Alix-Panabieres C. EpCAM-independent enrichment and detection of viable circulating tumor cells using the EPISPOT assay. Methods Mol Biol. 2017;1634:263–76.
Eyer K, et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. Nat Biotechnol. 2017;35:977–82.
Babayan A, et al. Comparative study of whole genome amplification and next generation sequencing performance of single cancer cells. Oncotarget. 2016;8:56066–80.
Maheswaran S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–77.
Jiang Y, Palma JF, Agus DB, Wang Y, Gross ME. Detection of androgen receptor mutations in circulating tumor cells in castration resistant prostate cancer. Clin Chem. 2010;56:1492–5.
Schneck H, et al. Analysing the mutational status of PIK3CA in circulating tumor cells from metastatic breast cancer patients. Mol Oncol. 2013;7:976–86.
Meric-Bernstam F, et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin Cancer Res. 2019. https://doi.org/10.1158/1078-0432.CCR-18-2275.
Lohr JG, Adalsteinsson VA, Cibulskis K, Choudhury AD, Rosenberg M, CruzGordillo P, et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat Biotechnol. 2014;32:479–84.
Ni X, Zhuo M, Su Z, Duan J, Gao Y, Wang Z, et al. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc Natl Acad Sci USA. 2013;110:21083–8.
Carter L, Rothwell D, Mesquita B, et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive and chemorefractory small-cell lung cancer. Nat Med. 2017;23:114–9. https://doi.org/10.1038/nm.4239.
Heitzer E, et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 2013;73(10):2965–75.
Yu B, Li Y, Yuan H, Zhang B, Jiang X, Yu M, Zhu H, You Q, Wang L. Whole Genome Sequencing in single CTC improves clinical outcome in Her-2 negative breast cancer patients (2020). https://doi.org/10.21203/rs.3.rs-15473/v1.
Gorges TM, et al. Accession of tumor heterogeneity by multiplex transcriptome profiling of single circulating tumor cells. Clin Chem. 2016;62:1504–15.
Jordan NV, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537:102–6.
Antonarakis ES, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371:1028–38.
Steinestel J, et al. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget. 2015. https://doi.org/10.18632/oncotarget.
Antonarakis ES, et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1:582–91.
Nakazawa M, et al. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol. 2015;26:1859–65.
Onstenk W, et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015;68:939–45.
Scher HI, et al. Nuclear-specific AR-V7 protein localization is necessary to guide treatment selection in metastatic castration-resistant prostate cancer. Eur Urol. 2017;71:874–82.
Scher HI, et al. Assessment of the validity of nuclear-localized androgen receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. JAMA Oncol. 2018;4:1179–86.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4.
Ting DT, Wittner BS, Ligorio M, Vincent Jordan N, Shah AM, Miyamoto DT, et al. Single-cell RNA sequencing identifies extracellular matrix gene expression by pancreatic circulating tumor cells. Cell Rep. 2014;8:1905–18.
Miyamoto DT, et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351–6.
Stewart CA, Gay CM, Xi Y, et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nat Cancer. 2020. https://doi.org/10.1038/s43018-019-0020-z}.
Zhang Y, et al. Single-cell detection of metabolic activity, intracellular functional proteins, and genetic mutations from rare circulating tumor cells. Anal Chem. 2015;87:9761–8.
Yao X, et al. Functional analysis of single cells identifies a rare subset of circulating tumor cells with malignant traits. Integr Biol (Camb). 2014;6:388–98.
Sinkala E, et al. Profiling protein expression in circulating tumour cells using microfluidic western blotting. Nat Commun. 2017;8:14622.
Gerdtsson E, Pore M, Thiele JA, Gerdtsson AS, Malihi PD, Nevarez R, et al. Multiplex protein detection on circulating tumor cells from liquid biopsies using imaging mass cytometry. Converg Sci Phys Oncol. 2018. https://doi.org/10.1088/2057-1739/aaa013.
Franzen B, et al. A fine-needle aspiration-based protein signature discriminates benign from malignant breast lesions. Mol Oncol. 2018;12:1415–28.
Pixberg CF, Raba K, Müller F, Behrens B, Honisch E, Niederacher D, et al. Analysis of DNA methylation in single circulating tumor cells. Oncogene. 2017;36:3223–31.
Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176:98–112.
Hodgkinson CL, et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nat Med. 2014;20:897–903.
Baccelli I, et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol. 2013;31:539–44.
Alix-Panabieres C, et al. Molecular portrait of metastasis-competent circulating tumor cells in colon cancer reveals the crucial role of genes regulating energy metabolism and DNA repair. Clin Chem. 2017;63:700–13.
Gorges TM, et al. Enumeration and molecular characterization of tumor cells in lung cancer patients using a novel in vivo device for capturing circulating tumor cells. Clin Cancer Res. 2016;22:2197–206.
Soler A, et al. Autologous cell lines from circulating colon cancer cells captured from sequential liquid biopsies as model to study therapy-driven tumor changes. Sci Rep. 2018;8:15931.
Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355:1330–4.
Mostert B, et al. Gene expression profiles in circulating tumor cells to predict prognosis in metastatic breast cancer patients. Ann. Oncol. 2014;26:510–6.
Miyamoto DT, et al. Single-Cell Analysis of Circulating Tumor Cells as a Window into Tumor Heterogeneity. Cold Spring Harbor Symp Quant Biol. 2016;81:269–74. https://doi.org/10.1101/sqb.2016.81.031120.
Lambros MB, et al. Single-cell analyses of prostate cancer liquid biopsies acquired by apheresis. Clin Cancer Res. 2018;24:5635–44.
Paoletti C, et al. Comprehensive mutation and copy number profiling in archived circulating breast cancer tumor cells documents heterogeneous resistance mechanisms. Cancer Res. 2018;78:1110–22.
Pan H, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377:1836–46.
Sparano J, et al. Association of circulating tumor cells with late recurrence of estrogen receptor positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4:1700–6.
van Dalum G, et al. Importance of circulating tumor cells in newly diagnosed colorectal cancer. Int J Oncol. 2015;46:1361–8.
Lucci A, Hall CS, Patel SP, et al. Circulating tumor cells and early relapse in node-positive melanoma. Clin Cancer Res. 2020. https://doi.org/10.1158/1078-0432.ccr-19-2670.
Kantara C, O’Connell M, Luthra G, et al. Methods for detecting circulating cancer stem cells (CCSCs) as a novel approach for diagnosis of colon cancer relapse/metastasis. Lab Invest. 2015;95:100–12.
Bissolati M, Sandri MT, Burtulo G, et al. Portal vein-circulating tumor cells predict liver metastases in patients with resectable pancreatic cancer. Tumor Biol. 2015;36:991–6.
Chemi F, Rothwell DG, McGranahan N, Gulati S, Abbosh C, Pearce SP, et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat Med. 2019;25:1534–9.
Pantel K, Alix-Panabières C. Liquid biopsy and minimal residual disease—latest advances and implications for cure. Nat Rev Clin Oncol. 2019;16:409–24.
Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91.
Huang X, Gao P, Song Y, et al. Meta-analysis of the prognostic value of circulating tumor cells detected with the Cell Search System in colorectal cancer. BMC Cancer. 2015;15:202.
Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–9.
Foy V, Fernandez-Gutierrez F, Faivre-Finn C, et al. The clinical utility of circulating tumour cells in patients with small cell lung cancer. Transl Lung Cancer Res. 2017;6:409–17.
Lindsay CR, Blackhall FH, Carmel A, et al. EPAC-lung: pooled analysis of circulating tumour cells in advanced non-small cell lung cancer. Eur J Cancer. 2019;117:60–8.
Bidard FC, Huguet F, Louvet C, et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: the ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24:2057–61.
Zhang Y, Li J, Wang L, et al. Clinical significance of detecting circulating tumor cells in patients with esophageal squamous cell carcinoma by EpCAM-independent enrichment and immunostaining fluorescence in situ hybridization. Mol Med Rep. 2019;20:1551–60.
Riethdorf S, Hildebrandt L, Heinzerling L, et al. Detection and characterization of circulating tumor cells in patients with Merkel cell carcinoma. Clin Chem. 2019;65:462–72.
Abrahamsson J, Aaltonen K, Engilbertsson H, et al. Circulating tumor cells in patients with advanced urothelial carcinoma of the bladder: association with tumor stage, lymph node metastases, FDG-PET findings, and survival. Urol Oncol. 2017;35(606):606.e9–16.
Balakrishnan A, Koppaka D, Anand A, et al. Circulating Tumor Cell cluster phenotype allows monitoring response to treatment and predicts survival. Sci Rep. 2019;9:7933.
Mazel M, Jacot W, Pantel K, et al. Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol. 2015;9:1773–82.
Strati A, Koutsodontis G, Papaxoinis G, et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell carcinoma. Ann Oncol. 2017;28:1923–33.
Guibert N, Delaunay M, Lusque A, et al. PD-L1 expression in circulating tumor cells of advanced nonsmall cell lung cancer patients treated with nivolumab. Lung Cancer. 2018;120:108–12.
Saad N, Poudel A, Basnet A, Gajra A. Epidermal growth factor receptor T790M mutation-positive metastatic non-small-cell lung cancer: focus on osimertinib (AZD9291). Onco Targets Ther. 2017;10:1757–66.
Magbanua MJM, Rugo HS, Wolf DM, et al. Expanded genomic profiling of circulating tumor cells in metastatic breast cancer patients to assess biomarker status and biology over time (CALGB 40502 and CALGB 40503, Alliance). Clin Cancer Res. 2018;24:1486–99.
Lakhani S, Ellis I, Schnitt S, et al. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press; 2012.
Amin MB, Edge S, Greene F, et al. AJCC cancer staging manual. 8th ed. New York: Springer Publishing; 2017.
Cristofanilli M, Pierga JY, Reuben J, et al. The clinical use of circulating tumor cells (CTCs) enumeration for staging of metastatic breast cancer (MBC): international expert consensus paper. Crit Rev Oncol Hematol. 2019;134:39–45.
Kolostova K, Matkowski R, Jędryka M, et al. The added value of circulating tumor cells examination in ovarian cancer staging. Am J Cancer Res. 2015;5(11):3363–75.
Hou Y, Guo H, Cao C, et al. Single-cell triple omics sequencing reveals genetic, epigenetic, and transcriptomic heterogeneity in hepatocellular carcinomas. Cell Res. 2016;26:304–19. https://doi.org/10.1038/cr.2016.23.
Bian S, et al. Single-cell multiomics sequencing and analyses of human colorectal cancer. Science. 2018;362:1060–3.
Acknowledgements
We are thankful to Council of Scientific and Industrial Research, New Delhi for providing fellowship to AC [09/141(0209)/2019-EMR-1] and RK [09/141(0210)/2019-EMR-1]. The figures are drawn using Inkscape software and templates available from Servier Medical Art provided by Les Laboratoires Servier under Creative Commons Attribution 3.0 Unported License has been used.
Funding
Council of Scientific and Industrial Research, India for fellowship to Anshika Chauhan and Rajandeep Kaur.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chauhan, A., Kaur, R., Ghoshal, S. et al. Exploration of Circulating Tumour Cell (CTC) Biology: A Paradigm Shift in Liquid Biopsy. Ind J Clin Biochem 36, 131–142 (2021). https://doi.org/10.1007/s12291-020-00923-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-020-00923-4