Abstract
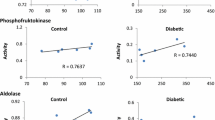
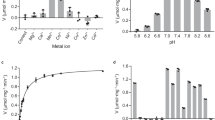
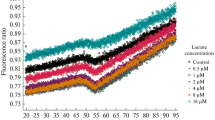
Recent studies have been noted that the erythrocytes from Type II diabetic patients show significantly altered structural and functional characteristics along with the changed intracellular concentrations of glycolytic intermediates. More recent studies from our laboratory have shown that the activities of enzymes of glycolytic pathway changed significantly in RBCs from Type II diabetic patients. In particular the levels of lactate dehydrogenase (LDH) increased significantly. Lactic acidosis is an established feature of diabetes and LDH plays a crucial role in conversion of pyruvate to lactate and reportedly, the levels of lactate are significantly high which is consistent with our observation on increased levels of LDH. Owing to this background, we examined the role of erythrocyte LDH in lactic acidosis by studying its kinetics properties in Type II diabetic patients. Km, Vmax and apparent catalytic efficiency were determined using pyruvate and NADH as the substrates. With pyruvate as the substrate the Km values were comparable but Vmax increased significantly in the diabetic group. With NADH as the substrate the enzyme activity of the diabetic group resolved in two components as against a single component in the controls. The Apparent Kcat and Kcat/Km values for pyruvate increased in the diabetic group. The Ki for pyruvate increased by two fold for the enzyme from diabetic group with a marginal decrease in Ki for NADH. The observed changes in catalytic attributes are conducive to enable the enzyme to carry the reaction in forward direction towards conversion of pyruvate to lactate leading to lactic acidosis.





Similar content being viewed by others
Abbreviations
- DM:
-
Diabetes mellitus
- HbA1c:
-
Glycosylated haemoglobin
- RBCs:
-
Red blood cells
- LDH:
-
Lactate dehydrogenase
- NADH:
-
Nicotinamide adenine dinucleotide
- G6P:
-
Glucose-6-phosphate
- F6P:
-
Fructose-6-phosphate
- FBP:
-
Fructose-1,6-bisphosphate
- DHAP:
-
Dihydroxyacetone phosphate
- GAP:
-
Glyceraldehyde 3-phosphate
- 3PGA:
-
3-Phosphoglyceric acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- NaCl:
-
Sodium chloride
References
Taylor SI, Kadowaki T, Kadowaki H, Accili D, Cama A, McKeon C. Mutations in insulin-receptor gene in insulin-resistant patients. Diabetes Care. 1990;13:257–79.
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:62–7.
Bunn HF. Evaluation of glycosylated hemoglobin in diabetic patients. Diabetes. 1981;30:613–7.
Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in non diabetic adults. N Engl J Med. 2010;362:800–11.
Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008;26:77–82.
Domingueti CP, Dusse LM, das Graças Carvalho M, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complic. 2016;30:738–45.
Shin S, Ku Y, Babu N, Singh M. Erythrocyte deformability and its variation in diabetes mellitus. Indian J Exp Biol. 2007;45:121.
Nayak BS, Beharry VY, Armoogam S, Nancoo M, Ramadhin K, Ramesar K, et al. Determination of RBC membrane and serum lipid composition in trinidadian type II diabetics with and without nephropathy. Vasc Health Risk Manag. 2008;4:893.
Buys AV, Van Rooy MJ, Soma P, Van Papendorp D, Lipinski B, Pretorius E. Changes in red blood cell membrane structure in type 2 diabetes: a scanning electron and atomic force microscopy study. Cardiovasc Diabetol. 2013;12:1.
Kono N, Kuwajima M, Tarui S. Alteration of glycolytic intermediary metabolism in erythrocytes during diabetic ketoacidosis and its recovery phase. Diabetes. 1981;30:346–53.
Mali AV, Bhise SS, Hegde MV, Katyare SS. Altered erythrocyte glycolytic enzyme activities in type-II diabetes. Indian J Clin Biochem. 2015:1–5.
Krzymień J, Karnafel W. Lactic acidosis in patients with diabetes. Pol Arch Med Wewn. 2013;123(3):91–7.
Lacticacidosis, Anasthesia, Acid Base. http://www.anaesthesia.com/AcidBase/ab8-1php/.
Buhl SN, Jackson KY, Lubinski R, Vanderlinde RE. Effect of reaction initiator on human lactate dehydrogenase assay. Clin Chem. 1976;22:1098–9.
Dixon M, Webb EC. Enzymes. London: Longman; 1979. p. 47–206.
Mathews CK, van Holde KE. Biochemistry. 2nd ed. Menlo Park: The Benjamin Cummings Publishing Co., Inc.; 1996.
Dave KR, Katyare SS. Effect of alloxan-induced diabetes on serum and cardiac butyrylcholinesterases in the rat. J Endocrinol. 2002;175:241–50.
Patel SP, Katyare SS. Insulin-status-dependent modulation of FoF1 ATPase activity in rat kidney mitochondria. Arch Physiol Biochem. 2006;112:150–7.
Jaenicke R, Knof S. Molecular weight and quaternary structure of lactic dehydrogenase. Eur J Biochem. 1968;4:157–63.
Quistorff B, Grunnet N. The isoenzyme pattern of LDH does not play a physiological role; except perhaps during fast transitions in energy metabolism. Aging. 2011;3:457–60.
Acknowledgements
This research was supported by funding from Bharati Vidyapeeth University, Pune.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Mali, A.V., Bhise, S.S., Katyare, S.S. et al. Altered Kinetics Properties of Erythrocyte Lactate Dehydrogenase in Type II Diabetic Patients and Its Implications for Lactic Acidosis. Ind J Clin Biochem 33, 38–45 (2018). https://doi.org/10.1007/s12291-017-0637-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12291-017-0637-6