Abstract
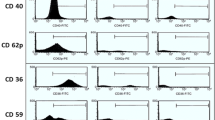
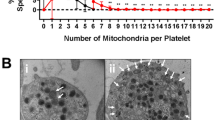
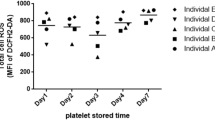
Hemolysis, a crucial feature of Sickle cell disease (SCD), is a key player for cellular activation leading to various complications including thrombosis. In response to hemolysis, platelets get activated and release components that are necessary for further platelet activation and aggregation. Thus, it is believed that platelets contribute to the development of thrombotic complications. Platelets in SCD are expected to be affected due to common cause of hemolysis. To measure the surface markers of platelets including P-Selectin, Phosphatidyl Serine and integrin αIIbβ3 in SCD patients and healthy controls in order to understand the status of the platelets in SCD. To measure the surface markers of activated platelets using flow cytometry. Since mitochondria and calcium play an important role in cellular functions, the mitochondrial membrane potential and calcium content of platelets in SCD were also evaluated using flow cytometry. In the present study, we have observed significant increase of calcium level in SCD platelets. Further, the loss of mitochondrial membrane potential in SCD platelets was found to be significantly higher when compared to platelets of healthy controls. Though the surface markers of activated platelets in SCD remain unchanged, increased level of calcium and mitochondrial membrane potential loss suggest that the platelets in SCD are more prone to become activated. In order to understand the status of the platelets in SCD, apart from the surface markers, it is also important to assess the calcium levels and mitochondrial membrane potential of platelets.





Similar content being viewed by others
References
Quinn CT (2016) Minireview: clinical severity in sickle cell disease: the challenges of definition and prognostication. Exp Biol Med 241(7):679–688. https://doi.org/10.1177/1535370216640385
Kavanagh PL, Fasipe TA, Wun T (2022) Sickle cell disease: a review. JAMA 328(1):57–68. https://doi.org/10.1001/jama.2022.10233
Rees DC, Williams TN, Gladwin MT (2010) Sickle-cell disease. Lancet (London, England) 376(9757):2018–2031. https://doi.org/10.1016/S0140-6736(10)61029-X
Annarapu GK, Nolfi-Donegan D, Reynolds, et al (2021) Mitochondrial reactive oxygen species scavenging attenuates thrombus formation in a murine model of sickle cell disease. JTH 19(9):2256–2262. https://doi.org/10.1111/jth.15298
Villagra J, Shiva S, Hunter LA et al (2007) Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood 110(6):2166–2172. https://doi.org/10.1182/blood-2006-12-061697
Wun T, Paglieroni T, Rangaswami A et al (1998) Platelet activation in patients with sickle cell disease. Br J Haematol 100(4):741–749. https://doi.org/10.1046/j.1365-2141.1998.00627.x
Vogel S, Thein SL (2018) Platelets at the crossroads of thrombosis, inflammation and haemolysis. Br J Haematol 180(5):761–767. https://doi.org/10.1111/bjh.15117
Frelinger AL 3rd, Jakubowski JA, Brooks JK et al (2014) Platelet activation and inhibition in sickle cell disease (pains) study. Platelets 25(1):27–35. https://doi.org/10.3109/09537104.2013.770136
Osman Y, Vatte CB (2018) Study of platelet activation markers and plasma cytokines in sickle cell disease patients during vaso-occlusive pain crises. J Hematopathol 11:37–44. https://doi.org/10.1007/s12308-018-0322-6
Thein MS, Igbineweka NE, Thein SL (2017) Sickle cell disease in the older adult. Pathology 49(1):1–9. https://doi.org/10.1016/j.pathol.2016.10.002
Yamakawa K, Ogura H, Koh T et al (2013) Platelet mitochondrial membrane potential correlates with severity in patients with systemic inflammatory response syndrome. J Trauma Acute Care Surg 74(2):411–418. https://doi.org/10.1097/TA.0b013e31827a34cf
Kraemer BF, Hennis I, Karge A et al (2022) Platelet mitochondrial membrane depolarization reflects disease severity in patients with preeclampsia. Mol Med (Camb, MA) 28(1):51. https://doi.org/10.1186/s10020-022-00472-x
Gründler K, Angstwurm M, Hilge R et al (2014) Platelet mitochondrial membrane depolarization reflects disease severity in patients with sepsis and correlates with clinical outcome. Crit Care (Lond, Engl) 18(1):R31. https://doi.org/10.1186/cc13724
Kaiser R, Escaig R, Kranich J et al (2022) Procoagulant platelet sentinels prevent inflammatory bleeding through GPIIBIIIA and PVI. Blood 140(2):121–139. https://doi.org/10.1182/blood.2021014914
Jardín I, López JJ, Pariente JA et al (2008) Intracellular calcium release from human platelets: different messengers for multiple stores. Trends Cardiovasc Med 18(2):57–61. https://doi.org/10.1016/j.tcm.2007.12.004
Kannan M, Ahmad F, Saxena R (2019) Platelet activation markers in evaluation of thrombotic risk factors in various clinical settings. Blood reviews 37:100583. https://doi.org/10.1016/j.blre.2019.05.007
Brass LF, Shattil SJ (1984) Identification and function of the high affinity binding sites for Ca2+ on the surface of platelets. J Clin Investig 73(3):626–632. https://doi.org/10.1172/JCI111252
Varga-Szabo D, Braun A, Nieswandt B (2009) Calcium signaling in platelets. J Thromb Haemost 7(7):1057–1066. https://doi.org/10.1111/j.1538-7836.2009.03455.x
Kannan M, Ahmad F, Yadav BK et al (2008) Carrier detection in Glanzmann thrombasthenia: comparison of flow cytometry and Western blot with respect to DNA mutation. Am J Clin Pathol 130(1):93–98. https://doi.org/10.1309/HYE4AP9961CEP0C0
Kannan M, Ahmad F, Yadav BK et al (2009) Molecular defects in ITGA2B and ITGB3 genes in patients with Glanzmann thrombasthenia. JTH 7(11):1878–1885. https://doi.org/10.1111/j.1538-7836.2009.03579.x
Jakubowski JA, Zhou C, Winters KJ et al (2015) The effect of prasugrel on ADP-stimulated markers of platelet activation in patients with sickle cell disease. Platelets 26(5):474–479. https://doi.org/10.3109/09537104.2014.940887
Berney SI, Ridler CD, Stephens AD et al (1992) Enhanced platelet reactivity and hypercoagulability in the steady state of sickle cell anaemia. Am J Hematol 40(4):290–294. https://doi.org/10.1002/ajh.2830400409
Tomer A, Harker LA, Kasey S et al (2001) Thrombogenesis in sickle cell disease. J Lab Clin Med 137(6):398–407. https://doi.org/10.1067/mlc.2001.115450
Brunson A, Keegan T, Mahajan A et al (2019) High incidence of venous thromboembolism recurrence in patients with sickle cell disease. Am J Hematol 94(8):862–870. https://doi.org/10.1002/ajh.25508
Naik RP, Streiff MB, Haywood C et al (2014) Venous thromboembolism incidence in the cooperative study of sickle cell disease. JTH 12(12):2010–2016. https://doi.org/10.1111/jth.12744
Jedlička J, Kunc R, Kuncová J (2021) Mitochondrial respiration of human platelets in young adult and advanced age - Seahorse or O2k? Physiol Res https://doi.org/10.33549/physiolres.934812
Palacka P, Gvozdjáková A, Rausová Z et al (2021) Platelet mitochondrial bioenergetics reprogramming in patients with urothelial carcinoma. Int J Mol Sci 23(1):388. https://doi.org/10.3390/ijms23010388
Wang Z, Cai F, Hu L et al (2014) The role of mitochondrial permeability transition pore in regulating the shedding of the platelet GPIbα. Ectodomain. Platelets 25(5):373–381. https://doi.org/10.3109/09537104.2013.821604
Jobe SM, Wilson KM, Leo L et al (2008) Critical role for the mitochondrial permeability transition pore and cyclophilin D in platelet activation and thrombosis. Blood 111(3):1257–1265. https://doi.org/10.1182/blood-2007-05-092684
Cardenes N, Corey C, Geary L et al (2014) Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex V inhibition, which contributes to platelet activation. Blood 123(18):2864–2872. https://doi.org/10.1182/blood-2013-09-529420
Acknowledgements
The authors thank the funding agency, Science and Engineering Research Board, Department of Science and Technology (DST-SERB), Government of India, INDIA (Grant ID: EMR/2016/000955) for supporting this study. The authors also thank the participants who volunteered for this study.
Author information
Authors and Affiliations
Contributions
SM, CLR and KK collected samples, performed experiments and collected data. RR helped with the sample collection and clinical data collection. FA critically revised the manuscript for important intellectual content. RS and MM supported the clinical data, interpreted and validated the manuscript. MK designed the study, supervised the project, interpreted the data and wrote the manuscript. All authors have approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interests.
Ethical approval
This study was approved by the institutional human ethics committee (IEC-514). All procedures followed were in accordance with the ethical standards of the institutional committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008 (5). All persons gave their informed consent prior to their inclusion in the study. Informed consent was obtained from all patients for being included in the study.
Consent to participate
Informed consent had been taken from the participants of this study.
Consent for publication
Consent for publication had been taken from all the contributing authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maharana, S., Roy, C.L., Kishor, K. et al. Depolarized Mitochondrial Membrane Potential and Elevated Calcium in Platelets of Sickle Cell Disease. Indian J Hematol Blood Transfus 39, 565–571 (2023). https://doi.org/10.1007/s12288-023-01640-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-023-01640-7