Abstract
Background
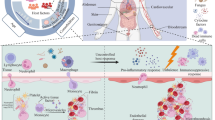
Sepsis is one of the main concerns of health and one of the leading causes of death in hospitals. It is essential to manage sepsis in hospitalized patients. In recent years, cell therapy has been considered as a new approach to treat sepsis. This study evaluated the effect of CXCR4 as one of the main proteins involved in the homing of mesenchymal stem cells in the sepsis serum in mice model.
Methods
Mouse sepsis model was induced by injection of E.coli and biochemical analyses was done to confirm the organ failure. Mesenchymal stem cells (MSCs) derived from bone marrow were separated into sepsis and control groups. In the sepsis serum group, MSCs were treated with sepsis serum at two time points: 24 and 48 h. Quantitative RT-PCR and flow cytometry were performed to determine the mRNA expression of CXCR4 in sepsis serum group compared to control group. Also, a migration assay was done to assess the migration capacity of bone marrow MSCs during inflammation and treatment in sepsis.
Results
Our result showed that treatment with sepsis serum can control migration by decrease in CXCR4 level (P ≤ 0.05) compared to control group. Moreover it was also reported that sepsis serum decreased mRNA expression of CXCR4 in MScs.
Conclusions
In our study, MSCs treated with septic serum were no longer able to migrate . Probably many variables such as source, dose, injection time, and injection route of MSCs after sepsis induction in the animal models are key factors for successful cell therapy.





Similar content being viewed by others
References
Wiersinga WJ et al (2014) Host innate immune responses to sepsis. Virulence 5(1):36–44
Schulte W, Bernhagen J, Bucala R (2013) Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediators of inflammation, 2013
Keane C, Jerkic M, Laffey JG (2017) Stem cell–based therapies for sepsis Anesthesiology 127(6):1017–1034
Laroye C et al (2017) Concise Review: Mesenchymal Stromal/Stem Cells: A New Treatment for Sepsis and Septic Shock? Stem Cells 35(12):2331–2339
Hao Q et al (2015) Study of Bone Marrow and Embryonic Stem Cell-Derived Human Mesenchymal Stem Cells for Treatment of Escherichia coli Endotoxin‐Induced Acute Lung Injury in Mice. Stem cells translational medicine 4(7):832–840
Mei SH et al (2010) Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med 182(8):1047–1057
Sohni A, Verfaillie CM (2013) Mesenchymal stem cells migration homing and tracking. Stem cells international, 2013
Haque N et al (2020) Role of the CXCR4-SDF1-HMGB1 pathway in the directional migration of cells and regeneration of affected organs. World J Stem Cells 12(9):938
Yellowley C (2013) CXCL12/CXCR4 signaling and other recruitment and homing pathways in fracture repair.BoneKEy reports, 2(3)
Bianchi ME, Mezzapelle R (2020) The chemokine receptor CXCR4 in cell proliferation and tissue regeneration. Front Immunol 11:2109
Kucia M et al (2006) The migration of bone marrow-derived non-hematopoietic tissue-committed stem cells is regulated in an SDF-1-, HGF-, and LIF-dependent manner. Archivum immunologiae et therapiae experimentalis. 54:121–1352
Kawaguchi N, Zhang T-T, Nakanishi T (2019) Involvement of CXCR4 in normal and abnormal development. Cells 8(2):185
Yang J-X et al (2015) CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem 290(4):1994–2006
Deng C et al (2014) Up-regulation of CXCR4 in rat umbilical mesenchymal stem cells induced by serum from rat with acute liver failure promotes stem cells migration to injured liver tissue. Mol Cell Biochem 396(1–2):107–116
Renckens R et al (2006) Endogenous tissue-type plasminogen activator is protective during Escherichia coli-induced abdominal sepsis in mice. J Immunol 177(2):1189–1196
Angus DC et al (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29(7):1303–1310
Lalu MM et al (2016) Evaluating mesenchymal stem cell therapy for sepsis with preclinical meta-analyses prior to initiating a first-. -human trial 5:e17850
Pedrazza L et al (2017) Mesenchymal stem cells decrease lung inflammation during sepsis, acting through inhibition of the MAPK pathway. Stem Cell Res Ther 8(1):1–14
Liang H et al (2019) Adipose-derived mesenchymal stem cells ameliorate acute liver injury in rat model of CLP induced-sepsis via sTNFR1. Exp Cell Res 383(1):111465
Pedrazza L et al (2014) Mesenchymal stem cells decrease splenocytes apoptosis in a sepsis experimental model. Inflamm Res 63(9):719–728
Omidi A et al (2013) Evaluation of protective effect of hydroalcoholic extract of Crocus sativus petals on preventing of gentamicin induced peliosis hepatis and hepatic telangiectasis in rats. J Birjand Univ Med Sci 19(4):455–462
Luo C-j et al (2014) Mesenchymal stem cells ameliorate sepsis-associated acute kidney injury in mice. Shock 41(2):123–129
Gao F et al (2020) Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci 255:117719
Sun X-Y et al (2020) Efficacy of mesenchymal stem cell therapy for sepsis: a meta-analysis of preclinical studies. Stem Cell Res Ther 11:1–10
Asano K, Yoshimura S, Nakane A (2015) Adipose tissue-derived mesenchymal stem cells attenuate staphylococcal enterotoxin A-induced toxic shock. Infect Immun 83(9):3490–3496
Galstian GM et al (2015) The results of the Russian clinical trial of mesenchymal stromal cells (MSCs) in severe neutropenic patients (pts) with septic shock (SS)(RUMCESS trial). Am Soc Hematology
Miller RJ, Banisadr G, Bhattacharyya BJ (2008) CXCR4 signaling in the regulation of stem cell migration and development. J Neuroimmunol 198(1):31–38
Wynn RF et al (2004) A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood 104(9):2643–2645
Ma J et al (2015) Transplanted hUCB-MSCs migrated to the damaged area by SDF-1/CXCR4 signaling to promote functional recovery after traumatic brain injury in rats. 37:50–561
Chiriac A et al (2010) SDF-1-enhanced cardiogenesis requires CXCR4 induction in pluripotent stem cells. 3:674–6826
Alsayed Y et al (2007) Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)–dependent migration and homing in multiple myeloma. 109:2708–27177
Haro C, Medina M (2019) Lactobacillus casei CRL 431 improves endothelial and platelet functionality in a pneumococcal infection model. Beneficial microbes 10(5):533–541
Kim HK et al (2007) Surface expression of neutrophil CXCR4 is down-modulated by bacterial endotoxin. Int J Hematol 85(5):390
Acknowledgements
The present work was supported financially by Shahroud University of Medical Sciences ( Grant No.9828).
Funding
The present work was supported financially by Shahroud University of Medical Sciences (Grant No.9828).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghanbari, M.A., Lashkar Bolouki, T., Norouzi, P. et al. Down-Regulation of CXCR4 in Mesenchymal Stem Cells by Septic Serum. Indian J Hematol Blood Transfus 38, 718–725 (2022). https://doi.org/10.1007/s12288-022-01560-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-022-01560-y