Abstract
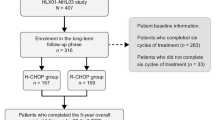
Diffuse large B cell lymphoma (DLBCL) is the most common form of non-Hodgkin lymphoma among adults, although it also affects the young and the elderly. DLBCL is treated with a chimeric monoclonal antibody against CD20, a B cell surface protein, named rituximab, in combination with a multidrug chemotherapeutic regimen. However, owing to its high cost, rituximab cannot be afforded by patients in developing or underdeveloped countries. In such cases, biosimilars of rituximab have been used instead of rituximab, with equivalent efficacy. In this single center, retrospective, observational study, we have compared patient outcomes such complete response (CR), partial response (PR), and overall response rate (ORR) in a cohort of 152 patients in an Indian hospital, who were treated either with innovator rituximab or Reditux, a biosimilar. We observed that the ORRs of both groups (88% in innnovator group and 82% in biosimilar group) were comparable. There was no statistically significant difference between the two groups in terms of CR (p = 0.353), PR (p = 0.42), ORR (p = 0.23), unfavorable responses, and stable or progressive disease (p = 0.42). The number of patients who died due to complications were few, and there was no significant difference between the two groups. The differences in the 3-year event-free survival and overall survival were not statistically significant. Biosimilar rituximab can suitably and safely replace the innovator rituximab for treatment of diffuse large B cell lymphoma.


Similar content being viewed by others
References
(1997) A clinical evaluation of the international lymphoma study group classification of non-Hodgkin’s lymphoma. Blood 89(11):3909
World Cancer Report (2014) International agency for research on cancer. Accessed July 2014
Coiffier B, Thieblemont C, Van Den Neste E et al (2010) Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 116(12):2040–2045
Pfreundschuh M, Kuhnt E, Trumper L et al (2011) CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 12(11):1013–1022
Bosch X, Ramos-Casals M, Khamashta MA (2013) Drugs targeting B-cells in autoimmune diseases. Springer Science & Business Media, Berlin, pp 1–4
Bloomberg (2007) Dr Reddy’s generic of Roche’s cancer drug to cost 50% less. http://www.livemint.com/Companies/K45flhIll1QCia18FMT5UK/Dr-Reddys-generic-of-Roches-cancer-drug-to-cost-50-less.html. Accessed 02 Feb 2017
Qureshi ZP, Magwood JS, Singh S, Bennett CL (2013) Rituximab and biosimilars—equivalence and reciprocity. Biosimilars 3:19–25
Cheson BD, Fisher RI, Barrington SF (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3067
The Lancet Haematology (2017) Biosimilars: an optimistic outlook, but vigilance is needed. Lancet Haematol 4(8):e341
Gulácsi L, Brodszky V, Baji P, Rencz F, Péntek M (2017) The rituximab biosimilar CT-P10 in rheumatology and cancer: a budget impact analysis in 28 European countries. Adv Ther 34(5):1128–1144. https://doi.org/10.1007/s12325-017-0522-y(Epub 2017 Apr 10)
Cohen S, Emery P, Greenwald M, Yin D, Becker JC, Melia LA, Li R, Gumbiner B, Thomas D, Spencer-Green G, Meng X (2016) A phase I pharmacokinetics trial comparing PF-05280586 (a potential biosimilar) and rituximab in patients with active rheumatoid arthritis. Br J Clin Pharmacol 82(1):129–138. https://doi.org/10.1111/bcp.12916(Epub 2016 Apr 29)
Yoo DH, Suh CH, Shim SC, Jeka S, Molina FFC, Hrycaj P, Wiland P, Lee EY, Medina-Rodriguez FG, Shesternya P, Radominski S, Stanislav M, Kovalenko V, Sheen DH, Myasoutova L, Lim MJ, Choe JY, Lee SJ, Lee SY, Kim SH, Park W (2017) Efficacy, safety and pharmacokinetics of up to two courses of the rituximab biosimilar CT-P10 versus innovator rituximab in patients with rheumatoid arthritis: results up to week 72 of a phase i randomized controlled trial. BioDrugs 31(4):357–367. https://doi.org/10.1007/s40259-017-0232-7
Kim WS, Buske C, Ogura M, Jurczak W, Sancho JM, Zhavrid E, Kim JS, Hernández-Rivas JÁ, Prokharau A, Vasilica M, Nagarkar R, Osmanov D, Kwak LW, Lee SJ, Lee SY, Bae YJ, Coiffier B (2017) Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 compared with rituximab in patients with previously untreated advanced-stage follicular lymphoma: a randomised, double-blind, parallel-group, non-inferiority phase 3 trial. Lancet Haematol 4(8):e362–e373. https://doi.org/10.1016/S2352-3026(17)30120-5(Epub 2017 Jul 14)
Ogura M, Sancho JM, Cho SG, Nakazawa H, Suzumiya J, Tumyan G, Kim JS, Lennard A, Mariz J, Ilyin N, Jurczak W, Lopez Martinez A, Samoilova O, Zhavrid E, Yañez Ruiz E, Trneny M, Popplewell L, Coiffier B, Buske C, Kim WS, Lee SJ, Lee SY, Bae YJ, Kwak LW (2018) Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 in comparison with rituximab in patients with previously untreated low-tumour-burden follicular lymphoma: a randomised, double-blind, parallel-group, phase 3 trial. Lancet Haematol 5(11):e543–e553. https://doi.org/10.1016/S2352-3026(18)30157-1
Jurczak W, Moreira I, Kanakasetty GB, Munhoz E, Echeveste MA, Giri P, Castro N, Pereira J, Akria L, Alexeev S, Osmanov E, Zhu P, Alexandrova S, Zubel A, Harlin O, Amersdorffer J (2017) Rituximab biosimilar and reference rituximab in patients with previously untreated advanced follicular lymphoma (ASSIST-FL): primary results from a confirmatory phase 3, double-blind, randomised, controlled study. Lancet Haematol 4(8):e350–e361. https://doi.org/10.1016/S2352-3026(17)30106-0(Epub 2017 Jul 14)
Gota V, Karanam A, Rath S, Yadav A, Tembhare P, Subramanian P, Sengar M, Nair R, Menon H (2016) Population pharmacokinetics of Reditux™, a biosimilar rituximab, in diffuse large B-cell lymphoma. Cancer Chemother Pharmacol 78(2):353–359. https://doi.org/10.1007/s00280-016-3083-x(Epub 2016 Jun 21)
Roy PS, John S, Karankal S, Kannan S, Pawaskar P, Gawande J, Bagal B, Khattry N, Sengar M, Menon H, Gujral S, Nair R (2013) Comparison of the efficacy and safety of rituximab (mabthera™) and its biosimilar (reditux™) in diffuse large B-cell lymphoma patients treated with chemo-immunotherapy: a retrospective analysis. Indian J Med Paediatr Oncol 34(4):292–298. https://doi.org/10.4103/0971-5851.125248
Ganesan P, Sagar TG, Kannan K, Radhakrishnan V, Rajaraman S, John A, Sundersingh S, Mahajan V, Ganesan TS (2017) Long-term outcome of diffuse large B-cell lymphoma: impact of biosimilar rituximab and radiation. Indian J Cancer 54(2):430–435. https://doi.org/10.4103/ijc.ijc_241_17
Candelaria M, Gonzalez DE, Beniwal SK, Dasappa L, Bar DO, Delamain MT, Mukhopadhyay A, Flores DH, Bhurani D, Radhakrishnan V, Salvatierra A, Kowalyszyn RD, Lipatov O, Patel M, Schusterschitz S, Volodicheva E, Perez LA (2017) A randomized, double-blind, phase III study comparing proposed biosimilar rituximab (RTXM83) versus reference rituximab, both in combination with CHOP, in the first line treatment of patients with diffuse large B-cell lymphoma (DLBCL). Blood 130(Suppl 1):1556
Jayakrishnan T, Jeeja MC, Kuniyil V, Paramasivam S (2016) Increasing out-of-pocket health care expenditure in India-due to supply or demand? Pharmacoeconomics 1:105. https://doi.org/10.4172/pe.1000105
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Human and Animal Rights
A formal consent wavier was obtained in view of the retrospective nature of the study. No identifiable human data were used for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bankar, A., Korula, A., Abraham, A. et al. Comparison of the Efficacy of Innovator Rituximab and its Biosimilars in Diffuse Large B Cell Lymphoma Patients: A Retrospective Analysis. Indian J Hematol Blood Transfus 36, 71–77 (2020). https://doi.org/10.1007/s12288-019-01167-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-019-01167-w