Abstract
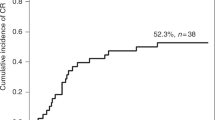
Twelve adult patients (median age 29.5 years) were started on Eltrombopag 25–50 mg/day for post-hematopoietic stem cell transplantation (HSCT) thrombocytopenia. All patients were having primary thrombocytopenia after HSCT. No patient had other secondary cause for thrombocytopenia. Two patients were allogenic subsets (1 acute myeloid leukemia i.e., AML and 1 aplastic anemia), and 10 were autologous transplants (3 multiple myeloma, 6 lymphoma and 1 AML). Nine patients were males, three were females. The median time of starting Eltrombopag was 21 days post-stem cell infusion (range day +17 to +60) at a median platelet count of 9,000/cmm (range 3,000–11,000/cmm). The median duration for treatment was 29 days. Median total dose of 812.5 mg was received by patients and they had a median platelet increment of 36,000/cmm. We observed that there were no adverse effects in these patients and there was a gradual increase in platelet count so that none of the patients had any complication due to thrombocytopenia. The cost of treatment was less than the cost of extended hospitalization and irradiated single donor platelet transfusion.

Similar content being viewed by others
References
Liesveld JL, Phillips II GL, Becker MW, Constine L, Friedberg JW, Andolina J, Milner L, Smudzin T, Hyrien O, Dawson KL, Chen Y (2012) Phase I study of eltrombopag for promoting thrombopoiesis in patients undergoing stem cell transplantation after total body irradiation. Oral and poster abstracts. In: 54th ASH annual meeting and exposition
Chawla SP, Staddon A, Hendifar A, Messam CA, Patwardhan R, Kamel YM (2013) Results of a phase I dose escalation study of Eltrombopag in patients with advanced soft tissue sarcoma receiving doxorubicin and ifosphamide. BMC Cancer 13:121
Australian public assessment report for eltrombopagolamine (2010)
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raut, S.S., Shah, S.A., Sharanangat, V.V. et al. Safety and Efficacy of Eltrombopag in Post-hematopoietic Stem Cell Transplantation (HSCT) Thrombocytopenia. Indian J Hematol Blood Transfus 31, 413–415 (2015). https://doi.org/10.1007/s12288-014-0491-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-014-0491-0