Abstract
Background
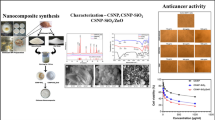
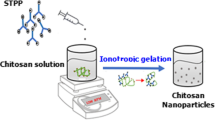
Allyl isothiocyanate (AITC) is an excellent active phytoconstituent recently revealed for cancer treatment. The strategic prominence of this study was to synthesize and characterize AITC-embedded tripolyphosphate-modified chitosan nanoparticles (AITC@CS-TPP-NPs) by ionic gelation.
Method
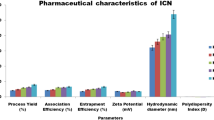
Chitosan is recycled as a polymer to fabricate AITC@CS-TPP-NPs; the fabricated nanoparticles (NPs) are then characterized using FT-IR spectroscopy, DSC, XRD, zeta potential, size analysis, SEM, EDX, entrapment efficiency, in vitro drug release study, and in vitro cytotoxicity activity against MCF-7 to explore the effectiveness and strength.
Results
As a result, developed AITC@CS-TPP-NPs demonstrates good stability with a zeta potential of 35.83 mV and 90.14% of drug release. The anticancer potential of AITC@CS-TPP-NPs shows the improved cytotoxicity activity of AITC due to the surface modification of CS using TPP. Hence, the cytotoxicity of AITC@CS-TPP-NPs was tested in vitro against a human breast cancer cell line (MCF-7) and found to be considerable.
Conclusion
The AITC@CS-TPP-NPs were effectively synthesized and have significant benefits, including being easy to prepare, stable, and affordable with wide use in human breast cancer against cell line (MCF-7).
Graphical abstract












Similar content being viewed by others
Data availability
Not applicable.
References
Ballauff M, Lu Y. “Smart” nanoparticles: preparation, characterization and applications. Polymer. 2007;48(7):1815–23.
Sawtarie N, Cai Y, Lapitsky Y. Preparation of chitosan/tripolyphosphate nanoparticles with highly tunable size and low polydispersity. Colloids Surf, B. 2017;157:110–7.
Najminejad Z, et al. Clinical perspective: antibody-drug conjugates (ADCs) for the treatment of HER2-positive breast cancer. Mol Ther. 2023. https://doi.org/10.1016/j.ymthe.2023.03.019.
Debela DT, et al. New approaches and procedures for cancer treatment: current perspectives. SAGE Open med. 2021;9:20503121211034370.
Moo T-A, et al. Overview of breast cancer therapy. PET Clin. 2018;13(3):339–54.
Schirrmacher V. From chemotherapy to biological therapy: a review of novel concepts to reduce the side effects of systemic cancer treatment. Int J Oncol. 2019;54(2):407–19.
Mandracchia D, Tripodo G. Micro and nano-drug delivery systems. London: The Royal Society of Chemistry; 2021. p. 1–24.
Mohammed MA, et al. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017;9(4):53.
Lepeltier E, Bourgaux C, Couvreur P. Nanoprecipitation and the “Ouzo effect”: application to drug delivery devices. Adv Drug Deliv Rev. 2014;71:86–97.
Ahmed TA, Aljaeid BM. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des Dev Ther. 2016;10:483.
Matshetshe KI, et al. Preparation, characterization and in vitro release study of β-cyclodextrin/chitosan nanoparticles loaded Cinnamomum zeylanicum essential oil. Int J Biol Macromol. 2018;118:676–82.
Jonassen H, Kjøniksen A-L, Hiorth M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromol. 2012;13(11):3747–56.
Patil PB, Patel JK. Chemopreventive aspects, investigational anticancer applications and current perspectives on allyl isothiocyanate (AITC): a review. Mol Cell Biochem. 2023. https://doi.org/10.1007/s11010-023-04697-0.
Rajakumar T, et al. Effect of allyl isothiocyanate on NF-κB signaling in 7, 12-dimethylbenz (a) anthracene and N-methyl-N-nitrosourea-induced mammary carcinogenesis. Breast Cancer. 2018;25:50–9.
Chakraborty S, Bhattacharjee P. Supercritical carbon dioxide extraction of melatonin from Brassica campestris: in vitro antioxidant, hypocholesterolemic and hypoglycaemic activities of the extracts. Int J Pharm Sci Res. 2017;8(6):2486–95.
Ghadi A, et al. Synthesis and optimization of chitosan nanoparticles: Potential applications in nanomedicine and biomedical engineering. Caspian J Intern Med. 2014;5(3):156.
Esquivel R, et al. Synthesis and characterization of new thiolated chitosan nanoparticles obtained by ionic gelation method. Int J Polym Sci. 2015. https://doi.org/10.1155/2015/502058.
Joseph JJ, Sangeetha D, Gomathi T. Sunitinib loaded chitosan nanoparticles formulation and its evaluation. Int J Biol Macromol. 2016;82:952–8.
Ahmad MZ, et al. Progress in nanomedicine-based drug delivery in designing of chitosan nanoparticles for cancer therapy. Int J Polym Mater Polym Biomater. 2022;71(8):602–23.
Encinas-Basurto D, et al. Poly (lactic-co-glycolic acid) nanoparticles for sustained release of allyl isothiocyanate: characterization, in vitro release and biological activity. J Microencapsul. 2017;34(3):231–42.
Singh A, Mittal A, Benjakul S. Chitosan nanoparticles: preparation, food applications and health benefits. Sci Asia. 2021;47:1–10.
Perera U, Rajapakse N. Chitosan nanoparticles: preparation, characterization, and applications. In: Seafood processing by-products. Springer; 2014. p. 371–87.
Bugnicourt L, Ladavière C. Interests of chitosan nanoparticles ionically cross-linked with tripolyphosphate for biomedical applications. Prog Polym Sci. 2016;60:1–17.
Kong J, Yu S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin. 2007;39(8):549–59.
Khan ZG, Vaidehi BS, Sarang GN, Shivam GB, Patil PB. Second Order Derivative Spectrophotometric Method for Estimation of Lurasidone in Bulk and Pharmaceutical Formulation. J. Pharm Sci. Tech. 2017;7(1):02–5.
Pandey AP, et al. Nanoarchitectonics in cancer therapy and imaging diagnosis. J Nanosci Nanotechnol. 2014;14(1):828–40.
Pham DT, Saelim N, Tiyaboonchai W. Crosslinked fibroin nanoparticles using EDC or PEI for drug delivery: physicochemical properties, crystallinity and structure. J Mater Sci. 2018;53(20):14087–103.
Caputo F, et al. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J Controll Release. 2019;299:31–43.
Hosseini SF, et al. Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohyd Polym. 2013;95(1):50–6.
Nair RS, et al. An evaluation of curcumin-encapsulated chitosan nanoparticles for transdermal delivery. AAPS PharmSciTech. 2019;20(2):1–13.
D’Souza S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv Pharm. 2014. https://doi.org/10.1155/2014/304757
Houghton P, et al. The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods. 2007;42(4):377–87.
Wen L-L, et al. Quantitative detection of selenate-reducing bacteria by real-time PCR targeting the selenate reductase gene. Enzyme Microb Technol. 2016;85:19–24.
Garg U, et al. Current advances in chitosan nanoparticles based drug delivery and targeting. Adv Pharma Bull. 2019;9(2):195.
Cai L, et al. β-Cyclodextrin-allyl isothiocyanate complex as a natural preservative for strand-based wood composites. Compos B Eng. 2020;193: 108037.
Encinas-Basurto D, et al. Targeted drug delivery via human epidermal growth factor receptor for sustained release of allyl isothiocyanate. Curr Top Med Chem. 2018;18(14):1252–60.
Kim W-T, et al. Characterization of calcium alginate and chitosan-treated calcium alginate gel beads entrapping allyl isothiocyanate. Carbohyd Polym. 2008;71(4):566–73.
Bansal P, et al. In vitro anticancer activity of dietary bioagent (isothiocyanates) on HepG2 and B16F10 cell lines: a comparative study. Ann Plant Sci. 2013;2(7):234–7.
Pawlik A, et al. Sulforaphene, an isothiocyanate present in radish plants, inhibits proliferation of human breast cancer cells. Phytomedicine. 2017;29:1–10.
Acknowledgements
The authors express acknowledgment of their gratitude to the Management and Dean of the Faculty of Pharmacy, Nootan Pharmacy College, Sankalchand Patel University, Visnagar (Gujarat), as well as the Management and Principal of the H. R. Patel Institute of Pharmaceutical Education & Research, Shirpur (Maharashtra) for providing the necessary resources to complete the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Patil, P.B., Patel, J.K. Preparation, characterization, and in vitro cytotoxicity activity of allyl-isothiocyanate-embedded polymeric nanoparticles for potential breast cancer targeting. Breast Cancer 30, 1065–1078 (2023). https://doi.org/10.1007/s12282-023-01501-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01501-1