Abstract
Background
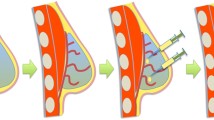
Based on the volume of tissue removed, conservative surgery (BCS) cannot always guarantee satisfactory cosmetic results, unless resorting to more complex oncoplastic approaches. Investigating an alternative to optimize aesthetic outcomes minimizing surgical complexity, was the purpose of this study. We assessed an innovative surgical procedure based on the use of a biomimetic polyurethane-based scaffold intended for regenerating soft-tissue resembling fat, in patients undergoing BCS for non-malignant breast lesions. Safety and performance of the scaffold, and safety and feasibility of the entire implant procedure were evaluated.
Methods
A volunteer sample of 15 female patients underwent lumpectomy with immediate device positioning, performing seven study visits with six-month follow-up. We evaluated incidence of adverse events (AEs), changes in breast appearance (using photographs and anthropomorphic measurements), interference with ultrasound and MRI (assessed by two independent investigators), investigator’s satisfaction (through a VAS scale), patient’s pain (through a VAS scale) and quality of life (QoL) (using the BREAST-Q© questionnaire). Data reported are the results of the interim analysis on the first 5 patients.
Results
No AEs were device related nor serious. Breast appearance was unaltered and the device did not interference with imaging. High investigator’s satisfaction, minimal post-operative pain and positive impact on QoL were also detected.
Conclusions
Albeit on a limited number of patients, data showed positive outcomes both in terms of safety and performance, paving the way to an innovative breast reconstructive approach with a potential remarkable impact on clinical application of tissue engineering.
Trial registration
ClinicalTrials.gov (NCT04131972, October 18, 2019).







Similar content being viewed by others
Data availability
The data presented in this study are available on request from the corresponding author.
References
American Cancer Society (2021). How common is breast cancer?, https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html; Accessed 15 Oct 2022
Ojala K, Meretoja TJ, Leidenius MH. Aesthetic and functional outcome after breast conserving surgery - Comparison between conventional and oncoplastic resection. Eur J Surg Oncol. 2017;43(4):658–64. https://doi.org/10.1016/j.ejso.2016.11.019.
Van Dongen JA, Voogd AC, Fentiman IS, Legrand C, Sylvester RJ, Tong D, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000;92(14):1143–50. https://doi.org/10.1093/jnci/92.14.1143.
Franceschini G, Martin Sanchez A, Di Leone A, Magno S, Moschella F, Accetta C, et al. New trends in breast cancer surgery: a therapeutic approach increasingly efficacy and respectful of the patient. G Chir. 2015;36(4):145–52. https://doi.org/10.11138/gchir/2015.36.4.145.
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32. https://doi.org/10.1056/NEJMoa020989.
Vidya R, Leff DR, Green M, McIntosh SA, St John E, Kirwan CC, et al. Innovations for the future of breast surgery. Br J Surg. 2021;108(8):908–16. https://doi.org/10.1093/bjs/znab147.
Behluli I, Le Renard PE, Rozwag K, Oppelt P, Kaufmann A, Schneider A. Oncoplastic breast surgery versus conventional breast-conserving surgery: a comparative retrospective study. ANZ J Surg. 2019;89(10):1236–41. https://doi.org/10.1111/ans.15245.
Tocchio A, Tamplenizza M, Martello F, Gerges I, Rossi E, Argentiere S, et al. Versatile fabrication of vascularizable scaffolds for large tissue engineering in bioreactor. Biomaterials. 2015;45:124–31. https://doi.org/10.1016/j.biomaterials.2014.12.031.
Tamplenizza M, Tocchio A, Gerges I, Martello F, Martelli C, Ottobrini L, et al. In Vivo Imaging Study of Angiogenesis in a Channelized Porous Scaffold. Mol Imaging. 2015. https://doi.org/10.2310/7290.2015.00011.
Alkhouli N, Mansfield J, Green E, Bell J, Knight B, Liversedge N, et al. The mechanical properties of human adipose tissues and their relationships to the structure and composition of the extracellular matrix. Am J Physiol Endocrinol Metab. 2013;305(12):E1427–35. https://doi.org/10.1152/ajpendo.00111.2013.
Rossi E, Gerges I, Tocchio A, Tamplenizza M, Aprile P, Recordati C, et al. Biologically and mechanically driven design of an RGD-mimetic macroporous foam for adipose tissue engineering applications. Biomaterials. 2016;104:65–77. https://doi.org/10.1016/j.biomaterials.2016.07.004.
Young DA, Choi YS, Engler AJ, Christman KL. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials. 2013;34(34):8581–8. https://doi.org/10.1016/j.biomaterials.2013.07.103.
Gerges I, Tamplenizza M, Martello F, Recordati C, Martelli C, Ottobrini L, et al. Exploring the potential of polyurethane-based soft foam as cell-free scaffold for soft tissue regeneration. Acta Biomater. 2018;73:141–53. https://doi.org/10.1016/j.actbio.2018.04.011.
Reginault P. Breast ptosis. Definition and treatment. Clin Plast Surg. 1976;3(2):193–203 (PMID: 1261176).
Cardoso MJ, Cardoso JS, Vrieling C, Macmillan D, Rainsbury D, Heil J, et al. Recommendations for the aesthetic evaluation of breast cancer conservative treatment. Breast Cancer Res Treat. 2012;135(3):629–37. https://doi.org/10.1007/s10549-012-1978-8.
Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–53. https://doi.org/10.1097/PRS.0b013e3181aee807.
Huss FR, Kratz G. Mammary epithelial cell and adipocyte co-culture in a 3-D matrix: the first step towards tissue-engineered human breast tissue. Cells Tissues Organs. 2001;169(4):361–7. https://doi.org/10.1159/000047903.
Morrison WA, Marre D, Grinsell D, Batty A, Trost N, O’Connor AJ. Creation of a Large Adipose Tissue Construct in Humans Using a Tissue-engineering Chamber: A Step Forward in the Clinical Application of Soft Tissue Engineering. EBioMedicine. 2016;6:238–45. https://doi.org/10.1016/j.ebiom.2016.03.032.
Wang X, Reagan MR, Kaplan DL. Synthetic adipose tissue models for studying mammary gland development and breast tissue engineering. J Mammary Gland Biol Neoplasia. 2010;15(3):365–76. https://doi.org/10.1007/s10911-010-9192-y.
Debels H. Advances in tissue engineering; a novel technology making use of an in vivo vascularized chamber. Acta Chir Belg. 2015;115(2):104–10. https://doi.org/10.1080/00015458.2015.11681078.
Chhaya MP, Melchels FP, Holzapfel BM, Baldwin JG, Hutmacher DW. Sustained regeneration of high-volume adipose tissue for breast reconstruction using computer aided design and biomanufacturing. Biomaterials. 2015;52:551–60. https://doi.org/10.1016/j.biomaterials.2015.01.025.
Chhaya MP, Balmayor ER, Hutmacher DW, Schantz JT. Transformation of Breast Reconstruction via Additive Biomanufacturing. Sci Rep. 2016;6:28030. https://doi.org/10.1038/srep28030.
Findlay MW, Dolderer JH, Trost N, Craft RO, Cao Y, Cooper-White J, et al. Tissue-engineered breast reconstruction: bridging the gap toward large-volume tissue engineering in humans. Plast Reconstr Surg. 2011;128(6):1206–15. https://doi.org/10.1097/PRS.0b013e318230c5b2.
Aimei J, Ming L, Wenjing D, Yilong D, Yanmei W. Improvement of the survival of human autologous fat transplantation by adipose-derived stem-cells-assisted lipotransfer combined with bFGF. Scie World J. 2015;2015:968057. https://doi.org/10.1155/2015/968057.
Wang L, Johnson JA, Zhang Q, Beahm EK. Combining decellularized human adipose tissue extracellular matrix and adipose-derived stem cells for adipose tissue engineering. Acta Biomater. 2013;9(11):8921–31. https://doi.org/10.1016/j.actbio.2013.06.035.
Cho SW, Song KW, Rhie JW, Park MH, Choi CY, Kim BS. Engineered adipose tissue formation enhanced by basic fibroblast growth factor and a mechanically stable environment. Cell Transplant. 2007;16(4):421–34. https://doi.org/10.3727/000000007783464795.
Cho SW, Kim SS, Rhie JW, Cho HM, Choi CY, Kim BS. Engineering of volume-stable adipose tissues. Biomaterials. 2005;26(17):3577–85. https://doi.org/10.1016/j.biomaterials.2004.09.013.
Rossi E, Guerrero J, Aprile P, Tocchio A, Kappos EA, Gerges I, et al. Decoration of RGD- mimetic porous scaffolds with engineered and devitalized extracellular matrix for adipose tissue regeneration. Acta Biomater. 2018;73:154–66. https://doi.org/10.1016/j.actbio.2018.04.039.
Xiao X, Zhou S, Wan J, Dong Z, Wang Y, Feng C. Pre-shaped large-volume engineered vascularized pedicled adipose flaps in a rabbit model: a two stage tissue engineering chamber-based procedure. J Biomater Tissue Eng. 2017;7:261–8. https://doi.org/10.1166/JBT.2017.1569.
Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Curcio CB, et al. A comparative translational study: The combined use of enhanced stromal vascular fraction and platelet-rich plasma improves fat grafting maintenance in breast reconstruction. Stem Cells Transl Med. 2012;1(4):341–51. https://doi.org/10.5966/sctm.2011-0065.
Tsuji W, Inamoto T, Ito R, Morimoto N, Tabata Y, Toi M. Simple and longstanding adipose tissue engineering in rabbits. J Artif Organs. 2013;16(1):110–4. https://doi.org/10.1007/s10047-012-0670-4.
Yao R, Zhang R, Lin F, Luan J. Biomimetic injectable HUVEC-adipocytes/collagen/alginate microsphere co-cultures for adipose tissue engineering. Biotechnol Bioeng. 2013;110(5):1430–43. https://doi.org/10.1002/bit.24784.
Rehnke RD, Schusterman MA 2nd, Clarke JM, Price BC, Waheed U, Debski RE, et al. Breast reconstruction using a three-dimensional absorbable mesh scaffold and autologous fat grafting: a composite strategy based on tissue-engineering principles. Plast Reconstr Surg. 2020;146(4):409e–13e. https://doi.org/10.1097/PRS.0000000000007172.
Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–6. https://doi.org/10.1016/S0140-6736(06)68438-9.
Fulco I, Miot S, Haug MD, Barbero A, Wixmerten A, Feliciano S, et al. Engineered autologous cartilage tissue for nasal reconstruction after tumour resection: an observational first-in-human trial. Lancet. 2014;384(9940):337–46. https://doi.org/10.1016/S0140-6736(14)60544-4.
Olausson M, Patil PB, Kuna VK, Chougule P, Hernandez N, Methe K, et al. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. Lancet. 2012;380(9838):230–7. https://doi.org/10.1016/S0140-6736(12)60633-3.
Raya-Rivera AM, Esquiliano D, Fierro-Pastrana R, López-Bayghen E, Valencia P, Ordorica-Flores R, et al. Tissue-engineered autologous vaginal organs in patients: a pilot cohort study. Lancet. 2014;384(9940):329–36. https://doi.org/10.1016/S0140-6736(14)60542-0.
Gerges I, Tamplenizza M, Martello F, Koman S, Chincarini C, Recordati C, et al. Conditioning the microenvironment for soft tissue regeneration in a cell free scaffold. Sci Rep. 2021;11:13310. https://doi.org/10.1038/s41598-021-92732-9.
Bulgheroni P, Bulgheroni E, Regazzola G, Mazzola C. Polyurethane scaffold for the treatment of partial meniscal tears Clinical results with a minimum two-year follow-up. Joints. 2014;1(4):161–6. https://doi.org/10.11138/jts/2013.1.4.161.
Acknowledgements
Authors kindly acknowledge all the colleagues and the operating room staff for organizational and technical support. Authors acknowledge support from the European Commission with the grant H2020-EIC-SMEInst-2018–2020 (Grant agreement number: 812002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MDM, BF, IG, MT, AT, FM, MG, and MR. The first draft of the manuscript was written by MDM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare the following financial/non-financial interests which may be considered as potential competing interests: Authors I.G., M.T., A.T. and F.M. are share-holders and members of the board of directors of Tensive S.r.l. They have received the grant H2020-EIC-SMEInst-2018–2020 (Grant agreement number: 812002) from the European Commission. The remaining authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study involving human participants, was conducted in accordance with the ethical standards of the institutional (AOUP Ethical Committee-Protocol # 51965, 11 October 2018) and national (Italian Ministry of Health-0066926-05/12/2018-DGDMF-MDS-P) research committee and with the 1964 Helsinki declaration and its later amendments and the Good Clinical Practice principles.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Mariniello, M.D., Ghilli, M., Favati, B. et al. Cell-free biomimetic polyurethane-based scaffold for breast reconstruction following non-malignant lesion resection. A first-in-human study. Breast Cancer 30, 559–569 (2023). https://doi.org/10.1007/s12282-023-01446-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01446-5