Abstract
Background
This study aimed to compare survival outcomes of neoadjuvant (NAC) and adjuvant chemotherapy (AdC) within each breast cancer subtype and stage among older women.
Methods
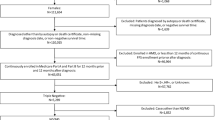
Older (≥ 66 years) women newly diagnosed with stage I–III invasive ductal breast cancer during 2010–2017 and treated with both chemotherapy and surgery within one year were identified from the Surveillance, Epidemiology, and End Results (SEER)-Medicare database. Analyses were performed within each of six groups, jointly defined based on subtype (hormone receptor [HR]-positive/human epidermal growth factor receptor 2 [HER2]-negative, HER2 + , and triple-negative) and stage (I–II and III). Kaplan–Meier curves and multivariable Cox models were used to compare overall and recurrence-free survival between NAC and AdC, with optimal full matching performed for confounding adjustment.
Results
Among 8,495 included patients, 8,329 (20.6% received NAC) remained after matching. Before multiple testing adjustment, Cox models showed that NAC was associated with a lower hazard for death among stage III HER2 + patients (hazard ratio = 0.347, 95% confidence interval CI 0.161–0.745) but a higher hazard for death among triple-negative patients (stage I–II: hazard ratio = 1.558, 95% CI 1.024–2.370; stage III: hazard ratio = 2.453; 95% CI 1.254–4.797). A higher hazard for death/recurrence was associated with NAC among stage I–II HR + /HER2– patients (hazard ratio = 1.305, 95% CI 1.007–1.693). No significant difference remained after multiple testing adjustment.
Conclusions
The opposite trends (before multiple testing adjustment) of survival comparisons for advanced HER2 + and triple-negative disease warrant further research. Caution is needed due to study limitations such as cancer stage validity.



Similar content being viewed by others
Data availability
The Surveillance, Epidemiology and End Results (SEER)-Medicare-linked data that support the findings of this study are available from the National Cancer Institute but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
References
Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: Triple-negative and HER2+ subtypes. Ann Surg Oncol. 2018;25:2241–8.
Mougalian SS, Soulos PR, Killelea BK, Lannin DR, Abu-Khalaf MM, DiGiovanna MP, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer Wiley. 2015;121:2544–52.
Puig CA, Hoskin TL, Day CN, Habermann EB, Boughey JC. National trends in the use of neoadjuvant chemotherapy for hormone receptor-negative breast cancer: A national cancer data base study. Ann Surg Oncol. 2017;24:1242–50.
Hoskin TL, Boughey JC, Day CN, Habermann EB. Lessons learned regarding missing clinical stage in the national cancer database. Ann Surg Oncol. 2019;26:739–45.
Zeidman M, Alberty-Oller JJ, Ru M, Pisapati KV, Moshier E, Ahn S, et al. Use of neoadjuvant versus adjuvant chemotherapy for hormone receptor-positive breast cancer: a national cancer database (NCDB) study. Breast Cancer Res Treat. 2020;184:203–12.
Zeidman M, Schmidt H, Alberty-Oller JJ, Pisapati KV, Ahn S, Mazumdar M, et al. Trends in neoadjuvant chemotherapy versus surgery-first in stage I HER2-positive breast cancer patients in the National Cancer DataBase (NCDB). Breast Cancer Res Treat. Springer Science and Business Media LLC; 2021;187:177–85.
Wang M, Hou L, Chen M, Zhou Y, Liang Y, Wang S, et al. Neoadjuvant chemotherapy creates surgery opportunities for inoperable locally advanced breast cancer. Sci Rep. 2017;7:44673.
Asselain B, Barlow W, Bartlett J, Bergh J, Bergsten-Nordström E, Bliss J, et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol Elsevier BV. 2018;19:27–39.
Mamounas EP, Fisher B. Preoperative (neoadjuvant) chemotherapy in patients with breast cancer. Semin Oncol Elsevier BV. 2001;28:389–99.
von Minckwitz G, Huang C-S, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med Massachusetts Medical Society. 2019;380:617–28.
Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med New England Journal of Medicine (NEJM/MMS); 2017;376:2147–59.
Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J Clin Oncol. 2021; JCO2003399.
Mauri D, Pavlidis N, Ioannidis JPA. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst: Oxford University Press (OUP); 2005.
Mieog JSD, van der Hage JA, van de Velde CJH. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. Wiley; 2007; CD005002.
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 2018 19:27–39.
Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. 2021;71:78–92.
Hutchins LF, Unger JM, Crowley JJ, Coltman CA Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7.
Mislang AR, Biganzoli L. Adjuvant systemic therapy in older breast cancer women: can we optimize the level of care? Cancers. 2015;7:1191–214.
Chubak J, Yu O, Pocobelli G, Lamerato L, Webster J, Prout MN, et al. Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. J Natl Cancer Inst: Oxford University Press (OUP); 2012.
Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36:1–10.
National Cancer Institute, Division of Cancer Control & Population Sciences. SEER-Medicare: Comorbidity SAS Macros [Internet]. [cited 2021 Oct 1]. Available from: https://healthcaredelivery.cancer.gov/seermedicare/considerations/calculation.html
Imai K, Ratkovic M. Covariate balancing propensity score. J R Stat Soc Series B Stat Methodol Wiley. 2014;76:243–63.
Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21.
Austin PC, Stuart EA. Optimal full matching for survival outcomes: a method that merits more widespread use. Stat Med Wiley. 2015;34:3949–67.
Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med Wiley. 2005;24:3089–110.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Wiley. 1995;57:289–300.
Zhang H, Barner JC, Moczygemba LR, Rascati KL, Park C, Kodali D. Neoadjuvant chemotherapy use trends among older women with breast cancer: 2010–2017. Breast Cancer Res Treat. 2022;193:695–705.
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from national surgical adjuvant breast and bowel project B-18. J Clin Oncol Am Soc Clin Oncol. 1997;15:2483–93.
Rubin M. When to adjust alpha during multiple testing: a consideration of disjunction, conjunction, and individual testing. Synthese. Springer Science and Business Media LLC; 2021;199:10969–1000.
Laas E, Bresset A, Féron J-G, Le Gal C, Darrigues L, Coussy F, et al. HER2-Positive breast cancer Patients with Pre-treatment axillary involvement or postmenopausal status benefit from neoadjuvant rather than adjuvant chemotherapy plus trastuzumab regimens. Cancers . Multidisciplinary Digital Publishing Institute; 2021; 13:370.
Pomponio MK, Burkbauer L, Goldbach M, Nazarian SM, Xie F, Clark AS, et al. Refining the indications for neoadjuvant chemotherapy for patients with HER2+ breast cancer: a single institution experience. J Surg Oncol Wiley. 2020;121:447–55.
Zheng S, Li L, Chen M, Yang B, Chen J, Liu G, et al. Benefits of neoadjuvant therapy compared with adjuvant chemotherapy for the survival of patients with HER2-positive breast cancer: a retrospective cohort study at FUSCC. Breast Elsevier BV. 2022;63:177–86.
Xia L-Y, Hu Q-L, Zhang J, Xu W-Y, Li X-S. Survival outcomes of neoadjuvant versus adjuvant chemotherapy in triple-negative breast cancer: a meta-analysis of 36,480 cases. World J Surg Oncol Springer Science and Business Media LLC; 2020; 18:129.
Giacchetti S, Hamy A-S, Delaloge S, Brai.n E, Berger F, Sigal-Zafrani B, et al. Long-term outcome of the REMAGUS 02 trial, a multicenter randomised phase II trial in locally advanced breast cancer patients treated with neoadjuvant chemotherapy with or without celecoxib or trastuzumab according to HER2 status. Eur J Cancer. 2017;75:323–32.
Amiri-Kordestani L, Wedam S, Zhang L, Tang S, Tilley A, Ibrahim A, et al. First FDA approval of neoadjuvant therapy for breast cancer: pertuzumab for the treatment of patients with HER2-positive breast cancer. Clin Cancer Res. American Association for Cancer Research (AACR); 2014;20:5359–64.
The Food and Drug Administration. FDA approves pembrolizumab for high-risk early-stage triple-negative breast cancer [Internet]. [cited 2022 Mar 3]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-high-risk-early-stage-triple-negative-breast-cancer
Ruhl J, Adamo M, Dickie L. SEER Program Coding and Staging Manual 2016: Section V [Internet]. National Cancer Institute, Bethesda, MD; 2020 Feb. Available from: https://seer.cancer.gov/archive/manuals/2016/SPCSM_2016_SectionV.pdf
Wu DY, Spangler AE, de Hoyos A, Vo DT, Seiler SJ. Quality of anatomic staging of breast carcinoma in hospitals in the United States, With focus on measurement of Tumor Dimension. Am J Clin Pathol. 2021;156:356–69.
Byrd DR, Brierley JD, Baker TP, Sullivan DC, Gress DM. Current and future cancer staging after neoadjuvant treatment for solid tumors. CA Cancer J Clin Wiley. 2021;71:140–8.
Fisher B, Gunduz N, Saffer EA. Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res. 1983;43:1488–92.
Acknowledgements
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results Program tumor registries in the creation of the SEER-Medicare database.
Funding
This study was funded by the PhRMA Foundation (2021 Predoctoral Fellowship Health Outcomes Research PDDS 846487).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Zhang, H., Barner, J.C., Moczygemba, L.R. et al. Comparing survival outcomes between neoadjuvant and adjuvant chemotherapy within breast cancer subtypes and stages among older women: a SEER-Medicare analysis. Breast Cancer 30, 489–496 (2023). https://doi.org/10.1007/s12282-023-01441-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-023-01441-w