Abstract
Background
There is a lack of data on combined radiotherapy (RT) and cyclin-dependent kinase 4 and 6 inhibitor (CDK4/6i) risk factors and toxicity. This study aimed to assess the incidence of and risk factors for non-hematologic toxicities in patients treated with combined RT and CDK4/6i using dose-volume parameter analysis.
Methods
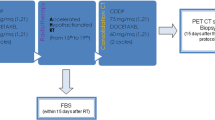
We conducted a retrospective multicenter cohort study of patients with metastatic breast cancer receiving RT within 14 days of CDK4/6i use. The endpoint was non-hematologic toxicities. Patient characteristics and RT treatment planning data were compared between the moderate or higher toxicities (≥ grade 2) group and the non-moderate toxicities group.
Results
Sixty patients were included in the study. CDK4/6i was provided at a median daily dose of 125 mg and 200 mg for palbociclib and abemaciclib, respectively. In patients who received concurrent RT and CDK4/6i (N = 29), the median concurrent prescribed duration of CDK4/6i was 14 days. The median delivered RT dose was 30 Gy and 10 fractions. The rate of grade 2 and 3 non-hematologic toxicities was 30% and 2%, respectively. There was no difference in toxicity between concurrent and sequential use of CDK4/6i. The moderate pneumonitis group had a larger lung V20 equivalent dose of 2 Gy per fraction and planning target volume than the non-moderate pneumonitis group.
Conclusions
Moderate toxicities are frequent with combined RT and CDK4/6i. Caution is necessary concerning the combined RT and CDK4/6i. Particularly, reducing the dose to normal organs is necessary for combined RT and CDK4/6i.

Similar content being viewed by others
Data availability
Raw data of this analysis is available from the corresponding author upon request.
Abbreviations
- AI:
-
Aromatase inhibitor
- BED:
-
Biologically effective dose
- CDK4/6i:
-
Cyclin-dependent kinase 4 and 6 inhibitors
- Dmax:
-
Maximum dose
- DVH:
-
Dose-volume histogram
- ECOG PS:
-
Eastern Cooperative Oncology Group performance status
- EQD2:
-
Equivalent dose of 2 Gy per fraction
- HER2− :
-
Human epidermal growth factor receptor 2 negative
- HR + :
-
Hormone receptor positive
- MLD:
-
Mean lung dose
- PTV:
-
Planning target volume
- RT:
-
Radiotherapy
References
Turner NC, Ro J, Andre F, Loi S, Verma S, Iwata H, et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–19.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29:1541–7.
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy-MONARCH 2: a randomized clinical trial. JAMA Oncol. 2020;6:116–24.
Fleming GF, Pagani O, Regan MM, Walley BA, Francis PA. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2022;33:658.
Chowdhary M, Sen N, Chowdhary A, Usha L, Cobleigh MA, Wang D, et al. Safety and efficacy of palbociclib and radiation therapy in patients with metastatic breast cancer: initial results of a novel combination. Adv Radiat Oncol. 2019;4:453–7.
Ippolito E, Greco C, Silipigni S, Dell’Aquila E, Petrianni GM, Tonini G, et al. Concurrent radiotherapy with palbociclib or ribociclib for metastatic breast cancer patients: preliminary assessment of toxicity. Breast. 2019;46:70–4.
Beddok A, Xu HP, Henry AA, Porte B, Fourquet A, Cottu P, et al. Concurrent use of palbociclib and radiation therapy: single-centre experience and review of the literature. Br J Cancer. 2020;123:905–8.
Guerini AE, Pedretti S, Salah E, Simoncini EL, Maddalo M, Pegurri L, et al. A single-center retrospective safety analysis of cyclin-dependent kinase 4/6 inhibitors concurrent with radiation therapy in metastatic breast cancer patients. Sci Rep. 2020;10:13589.
Kawamoto T, Shikama N, Sasai K. Severe acute radiation-induced enterocolitis after combined palbociclib and palliative radiotherapy treatment. Radiother Oncol. 2019;131:240–1.
Messer JA, Ekinci E, Patel TA, Teh BS. Enhanced dermatologic toxicity following concurrent treatment with palbociclib and radiation therapy: a case report. Rep Pract Oncol Radiother. 2019;24:276–80.
Nasir UM, Mozeika AM, Sayan M, Jan I, Kowal N, Haffty B, et al. Severe gastrointestinal mucositis following concurrent palbociclib and palliative radiation therapy. Anticancer Res. 2020;40:5291–4.
David S, Ho G, Day D, Harris M, Tan J, Goel S, et al. Enhanced toxicity with CDK 4/6 inhibitors and palliative radiotherapy: non-consecutive case series and review of the literature. Transl Oncol. 2021;14: 100939.
Lee CL, Oh P, Xu ES, Ma Y, Kim Y, Daniel AR, et al. Blocking cyclin-dependent kinase 4/6 during single dose versus fractionated radiation therapy leads to opposite effects on acute gastrointestinal toxicity in mice. Int J Radiat Oncol Biol Phys. 2018;102:1569–76.
Borst GR, Ishikawa M, Nijkamp J, Hauptmann M, Shirato H, Bengua G, et al. Radiation pneumonitis after hypofractionated radiotherapy: evaluation of the LQ(L) model and different dose parameters. Int J Radiat Oncol Biol Phys. 2010;77:1596–603.
Newcomb CH, Van Dyk J, Hill RP. Evaluation of isoeffect formulae for predicting radiation-induced lung damage. Radiother Oncol. 1993;26:51–63.
Barendsen GW. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys. 1982;8:1981–97.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transpl. 2013;48:452–8.
Ratosa I, Orazem M, Scoccimarro E, Steinacher M, Dominici L, Aquilano M, et al. Cyclin-dependent kinase 4/6 inhibitors combined with radiotherapy for patients with metastatic breast cancer. Clin Breast Cancer. 2020;20:495–502.
Kim KN, Shah P, Clark A, Freedman GM, Dastgheyb S, Barsky AR, et al. Safety of cyclin-dependent kinase4/6 inhibitor combined with palliative radiotherapy in patients with metastatic breast cancer. Breast. 2021;60:163–7.
Al-Rashdan A, Quirk S, Roumeliotis M, Abedin T, Amaro CP, Barbera L, et al. Radiation therapy with cyclin-dependent kinase 4/6 inhibitors: a multi-institutional safety and toxicity study. Int J Radiat Oncol Biol Phys. 2022. https://doi.org/10.1016/j.ijrobp.2022.07.005.
Yap YS, Kim SB, Chiu JWY, Lim E, Broom R, Liu Z, et al. 48P Abemaciclib combined with adjuvant endocrine therapy in patients from Asia with high risk early breast cancer: monarchE. Ann Oncol. 2021;32:S41–2.
Bosacki C, Bouleftour W, Sotton S, Vallard A, Daguenet E, Ouaz H, et al. CDK 4/6 inhibitors combined with radiotherapy: a review of literature. Clin Transl Radiat Oncol. 2021;26:79–85.
Fairchild A, Harris K, Barnes E, Wong R, Lutz S, Bezjak A, et al. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26:4001–11.
Sprave T, Verma V, Förster R, Schlampp I, Hees K, Bruckner T, et al. Quality of life and radiation-induced late toxicity following intensity-modulated versus three-dimensional conformal radiotherapy for patients with spinal bone metastases: results of a randomized trial. Anticancer Res. 2018;38:4953–60.
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–46.
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:2875–84.
Rugo HS, Diéras V, Gelmon KA, Finn RS, Slamon DJ, Martin M, et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: results from the PALOMA-2 trial. Ann Oncol. 2018;29:888–94.
Kalash R, Iarrobino NA, Beriwal S, Sun M, Glaser SM, Champ CE. Palbociclib enhances pulmonary fibrosis in patients undergoing thoracic radiation therapy: a case series and review of the literature. Int J Radiat Oncol Biol Phys. 2018;102: e610.
Funding
None.
Author information
Authors and Affiliations
Contributions
TK prepared the manuscript and conducted the literature search; TK reviewed and edited the manuscript; and TK, NS, NI, HK, TK, SS, HH, KY, YN, KM, YH, YW, AT, TS, NU, NA, and NN reviewed the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study was obtained from all patients. The study protocol was approved by the participating centers’ institutional review boards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kawamoto, T., Shikama, N., Imano, N. et al. Incidence of and risk factors for non-hematologic toxicity with combined radiotherapy and CDK4/6 inhibitors in metastatic breast cancer using dose-volume parameters analysis: a multicenter cohort study. Breast Cancer 30, 282–292 (2023). https://doi.org/10.1007/s12282-022-01422-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-022-01422-5