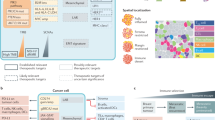
Abstract
Heterogeneity of the tumor microenvironment (TME) and the lack of a definite targetable receptor in triple-negative breast cancer (TNBC) has carved a niche for this cancer as a particularly therapeutically challenging form of breast cancer. However, recent advances in high-throughput genomic analysis have provided new insights into the unique microenvironment and defining characteristics of various subsets of TNBC. This improved understanding has contributed to the development of novel therapeutic strategies including targeted therapies such as PARP inhibitors and CDK inhibitors. Moreover, the recent FDA approval of the immune checkpoint inhibitor against programmed cell death protein 1 (PD-1), pembrolizumab and atezolizumab, holds the promise of improving the quality of life and increasing the overall survival of TNBC patients. This recent approval is one of the many therapeutically novel strategies that are currently being exploited in clinical trials toward eventual contribution to the oncologist’s toolbox against TNBC. In this review, we comprehensively discuss TNBC’s distinct TME and its immunophenotype. Furthermore, we highlight the histological and molecular classification of this cancer. More importantly, we describe how these characteristics and classifications contribute to the current standards of care and how they steer the development of newer and more targeted therapies toward achieving peak therapeutic goals in the treatment of TNBC.




Similar content being viewed by others
Data availability statement
The datasets/or images are available from the corresponding author upon reasonable request.
References
Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Fallahpour S, et al. Breast cancer survival by molecular subtype: a population-based analysis of cancer registry data. CMAJ Open. 2017;5(3):E734–9.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–48.
Huang S, et al. Integrative analysis reveals subtype-specific regulatory determinants in triple negative breast cancer. Cancers (Basel). 2019;11(4):507.
Goncalves H Jr, et al. Survival study of triple-negative and non-triple-negative breast cancer in a Brazilian cohort. Clin Med Insights Oncol. 2018;12:1179554918790563.
Xie N, et al. Clinicopathological characteristics and treatment strategies of triple-negative breast cancer patients with a survival longer than 5 years. Front Oncol. 2020;10: 617593.
Hancock BA, et al. Profiling molecular regulators of recurrence in chemorefractory triple-negative breast cancers. Breast Cancer Res. 2019;21(1):87.
Witzel I, et al. Treatment and outcomes of patients in the brain metastases in breast cancer network registry. Eur J Cancer. 2018;102:1–9.
Watson CJ, Khaled WT. Mammary development in the embryo and adult: new insights into the journey of morphogenesis and commitment. Development (Cambridge, England) 2020;147(22):169862
Anders C, Carey LA. Understanding and treating triple-negative breast cancer. Oncology (Williston Park). 2008;22(11):1233–9 (discussion 1239-40, 1243).
Van Keymeulen A, et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479(7372):189–93.
Kim MR, et al. TET2 directs mammary luminal cell differentiation and endocrine response. Nat Commun. 2020;11(1):4642.
Sarrio D, et al. Epithelial and mesenchymal subpopulations within normal basal breast cell lines exhibit distinct stem cell/progenitor properties. Stem Cells. 2012;30(2):292–303.
Bertucci F, et al. How basal are triple-negative breast cancers? Int J Cancer. 2008;123(1):236–40.
Lehmann BD, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67.
Prat A, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12(5):R68.
Deepak KGK, et al. Tumor microenvironment: challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol Res. 2020;153: 104683.
Wein L, et al. Clinical validity and utility of tumor-infiltrating lymphocytes in routine clinical practice for breast cancer patients: current and future directions. Front Oncol. 2017;7:156.
Zhang WJ, et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J Surg Res. 2018;222:93–101.
Zhou J, et al. Cancer-associated fibroblasts correlate with tumor-associated macrophages infiltration and lymphatic metastasis in triple negative breast cancer patients. J Cancer. 2018;9(24):4635–41.
Sahai E, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20(3):174–86.
He J, et al. Inhibition of USP2 eliminates cancer stem cells and enhances TNBC responsiveness to chemotherapy. Cell Death Dis. 2019;10(4):285.
Liubomirski Y, et al. Tumor-stroma-inflammation networks promote pro-metastatic chemokines and aggressiveness characteristics in triple-negative breast cancer. Front Immunol. 2019;10:757.
D’Esposito V, et al. Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5. Oncotarget. 2016;7(17):24495–509.
Liu L, et al. Cancer-associated adipocyte-derived G-CSF promotes breast cancer malignancy via Stat3 signaling. J Mol Cell Biol. 2020;12(9):723–37.
Lopatina T, et al. Targeting IL-3Ralpha on tumor-derived endothelial cells blunts metastatic spread of triple-negative breast cancer via extracellular vesicle reprogramming. Oncogenesis. 2020;9(10):90.
Kim J, et al. Heterogeneous perivascular cell coverage affects breast cancer metastasis and response to chemotherapy. JCI Insight. 2016;1(21): e90733.
Mirando AC, et al. Regulation of the tumor immune microenvironment and vascular normalization in TNBC murine models by a novel peptide. Oncoimmunology. 2020;9(1):1760685.
Sun H, et al. Cancer stem-like cells directly participate in vasculogenic mimicry channels in triple-negative breast cancer. Cancer Biol Med. 2019;16(2):299–311.
Bai J, et al. HIF-2alpha regulates CD44 to promote cancer stem cell activation in triple-negative breast cancer via PI3K/AKT/mTOR signaling. World J Stem Cells. 2020;12(1):87–99.
Maria Badowska-Kozakiewicz A, Piotr Budzik M. Triple-negative breast cancer: expression of hypoxia-inducible factor 1α in triple-negative breast cancer with metastasis to lymph nodes. Breast Cancer Surg 2018. Available: https://www.intechopen.com/chapters/60171, https://doi.org/10.5772/intechopen.75354
Lappano R, et al. The IL1beta-IL1R signaling is involved in the stimulatory effects triggered by hypoxia in breast cancer cells and cancer-associated fibroblasts (CAFs). J Exp Clin Cancer Res. 2020;39(1):153.
Alcaraz LB, et al. Cathepsin D exacerbates SPARC-driven aggressiveness by limited proteolysis in triple-negative breast cancer. bioRxiv 2020 p. 2020.10.22.350082.
Pawar A, Prabhu P. Nanosoldiers: a promising strategy to combat triple negative breast cancer. Biomed Pharmacother. 2019;110:319–41.
Mah EJ, et al. Collagen density modulates triple-negative breast cancer cell metabolism through adhesion-mediated contractility. Sci Rep. 2018;8(1):17094.
Wishart AL et al. Decellularized extracellular matrix scaffolds identify full-length collagen VI as a driver of breast cancer cell invasion in obesity and metastasis. Sci Adv 2020;6(43):3175
Xiong G, et al. Prolyl-4-hydroxylase alpha subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer. 2014;14:1.
Kuczek DE, et al. Collagen density regulates the activity of tumor-infiltrating T cells. J Immunother Cancer. 2019;7(1):68.
Kim H, et al. CTGF regulates cell proliferation, migration, and glucose metabolism through activation of FAK signaling in triple-negative breast cancer. Oncogene. 2021;40(15):2667–81.
Kala C, et al. Clinical and Cyto-morphological characterization of triple negative breast cancer. J Cytol. 2019;36(2):84–8.
Mills MN, et al. Histologic heterogeneity of triple negative breast cancer: a National Cancer Centre Database analysis. Eur J Cancer. 2018;98:48–58.
Liao HY, et al. The clinicopathological features and survival outcomes of different histological subtypes in triple-negative breast cancer. J Cancer. 2018;9(2):296–303.
Ishikawa Y, et al. Triple-negative breast cancer: histological subtypes and immunohistochemical and clinicopathological features. Cancer Sci. 2011;102(3):656–62.
Wang XX, et al. Difference in characteristics and outcomes between medullary breast carcinoma and invasive ductal carcinoma: a population based study from SEER 18 database. Oncotarget. 2016;7(16):22665–73.
Marchio C, Weigelt B, Reis-Filho JS. Adenoid cystic carcinomas of the breast and salivary glands (or “The strange case of Dr Jekyll and Mr Hyde” of exocrine gland carcinomas). J Clin Pathol. 2010;63(3):220–8.
Koo JS, Jung W. Clinicopathlogic and immunohistochemical characteristics of triple negative invasive lobular carcinoma. Yonsei Med J. 2011;52(1):89–97.
Rakha EA, et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. Eur J Cancer. 2008;44(1):73–83.
Choi HJ, et al. 265POncologic outcome of invasive lobular carcinoma: is it different from that of invasive ductal carcinoma? Annal Oncol. 2019;30(5):v90.
Li Y, et al. Comparative prognostic analysis for triple-negative breast cancer with metaplastic and invasive ductal carcinoma. J Clin Pathol. 2019;72(6):418–24.
Nelson RA, et al. Survival outcomes of metaplastic breast cancer patients: results from a US population-based analysis. Ann Surg Oncol. 2015;22(1):24–31.
Tsutsumi Y. Apocrine carcinoma as triple-negative breast cancer: novel definition of apocrine-type carcinoma as estrogen/progesterone receptor-negative and androgen receptor-positive invasive ductal carcinoma. Jpn J Clin Oncol. 2012;42(5):375–86.
Lehmann BD, Pietenpol JA. Clinical implications of molecular heterogeneity in triple negative breast cancer. Breast. 2015;24(Suppl 2):S36-40.
Prat A, et al. Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med. 2015;13:303.
Pareja F, et al. Triple-negative breast cancer: the importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer. 2016;2:16036.
Aslan M, et al. Oncogene-mediated metabolic gene signature predicts breast cancer outcome. npj Breast Cancer. 2021;7(1):141.
Nagayama A, et al. Novel antibody-drug conjugates for triple negative breast cancer. Ther Adv Med Oncol. 2020;12:1758835920915980.
Huang YH, et al. Expression pattern and prognostic impact of glycoprotein non-metastatic B (GPNMB) in triple-negative breast cancer. Sci Rep. 2021;11(1):12171.
Lehmann BD, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE. 2016;11(6): e0157368.
Burstein MD, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688–98.
Jezequel P, et al. Gene-expression molecular subtyping of triple-negative breast cancer tumours: importance of immune response. Breast Cancer Res. 2015;17:43.
Bareche Y, et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol. 2018;29(4):895–902.
Shangary S, Wang S. Targeting the MDM2-p53 interaction for cancer therapy. Clin Cancer Res. 2008;14(17):5318–24.
Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24(17):2899–908.
Shi Y, et al. Therapeutic landscape in mutational triple negative breast cancer. Mol Cancer. 2018;17(1):99.
Gao C, et al. Context-dependent roles of MDMX (MDM4) and MDM2 in breast cancer proliferation and circulating tumor cells. Breast Cancer Res. 2019;21(1):5.
Mayo LD, et al. PTEN protects p53 from Mdm2 and sensitizes cancer cells to chemotherapy. J Biol Chem. 2002;277(7):5484–9.
Wang D-Y, et al. A subgroup of microRNAs defines PTEN-deficient, triple-negative breast cancer patients with poorest prognosis and alterations in RB1, MYC, and Wnt signaling. Breast Cancer Res. 2019;21(1):18.
Hsieh JK, et al. RB regulates the stability and the apoptotic function of p53 via MDM2. Mol Cell. 1999;3(2):181–93.
Witkiewicz AK, Knudsen ES. Retinoblastoma tumor suppressor pathway in breast cancer: prognosis, precision medicine, and therapeutic interventions. Breast Cancer Res. 2014;16(3):207.
Liu L, et al. BRCAness as a prognostic indicator in patients with early breast cancer. Sci Rep. 2020;10(1):21173.
Tian T, et al. Evaluation of the BRCAness phenotype and its correlations with clinicopathological features in triple-negative breast cancers. Hum Pathol. 2019;84:231–8.
Chen H, et al. Association between BRCA status and triple-negative breast cancer: a meta-analysis. Front Pharmacol. 2018;9:909.
Costa R, et al. Targeting epidermal growth factor receptor in triple negative breast cancer: new discoveries and practical insights for drug development. Cancer Treat Rev. 2017;53:111–9.
Brand TM, et al. The nuclear epidermal growth factor receptor signaling network and its role in cancer. Discov Med. 2011;12(66):419–32.
Shim KG, et al. Inhibitory receptors induced by VSV viroimmunotherapy are not necessarily targets for improving treatment efficacy. Mol Ther. 2017;25(4):962–75.
Molloy NH, Read DE, Gorman AM. Nerve growth factor in cancer cell death and survival. Cancers (Basel). 2011;3(1):510–30.
Chakravarthy R, Mnich K, Gorman AM. Nerve growth factor (NGF)-mediated regulation of p75(NTR) expression contributes to chemotherapeutic resistance in triple negative breast cancer cells. Biochem Biophys Res Commun. 2016;478(4):1541–7.
Gao HF, et al. Prognostic significance of mesenchymal-epithelial transition in triple-negative breast cancers. Clin Breast Cancer. 2018;18(5):e961–6.
Pohl SG, et al. Wnt signaling in triple-negative breast cancer. Oncogenesis. 2017;6(4): e310.
Ireland L, et al. Blockade of insulin-like growth factors increases efficacy of paclitaxel in metastatic breast cancer. Oncogene. 2018;37(15):2022–36.
Davison Z, et al. Insulin-like growth factor-dependent proliferation and survival of triple-negative breast cancer cells: implications for therapy. Neoplasia. 2011;13(6):504–15.
Li CJ, et al. The molecular mechanism of epithelial-mesenchymal transition for breast carcinogenesis. Biomolecules. 2019;9(9):476.
Kim S, et al. Elevated TGF-beta1 and -beta2 expression accelerates the epithelial to mesenchymal transition in triple-negative breast cancer cells. Cytokine. 2015;75(1):151–8.
Cheung SY, et al. Role of epithelial-mesenchymal transition markers in triple-negative breast cancer. Breast Cancer Res Treat. 2015;152(3):489–98.
Vijay GV, et al. GSK3beta regulates epithelial-mesenchymal transition and cancer stem cell properties in triple-negative breast cancer. Breast Cancer Res. 2019;21(1):37.
Chen JH, et al. Upregulated SCUBE2 expression in breast cancer stem cells enhances triple negative breast cancer aggression through modulation of notch signaling and epithelial-to-mesenchymal transition. Exp Cell Res. 2018;370(2):444–53.
Riaz SK, et al. Influence of SHH/GLI1 axis on EMT mediated migration and invasion of breast cancer cells. Sci Rep. 2019;9(1):6620.
Rampurwala M, Wisinski KB, O’Regan R. Role of the androgen receptor in triple-negative breast cancer. Clin Adv Hematol Oncol. 2016;14(3):186–93.
Mina A, Yoder R, Sharma P. Targeting the androgen receptor in triple-negative breast cancer: current perspectives. Onco Targets Ther. 2017;10:4675–85.
Kong Y, et al. Effect of Bicalutamide on the proliferation and invasion of human triple negative breast cancer MDA-MB-231 cells. Medicine. 2020;99(17): e19822.
Stovgaard ES, et al. Triple negative breast cancer—prognostic role of immune-related factors: a systematic review. Acta Oncol. 2018;57(1):74–82.
Keren L, et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell. 2018;174(6):1373-1387.e19.
Lv Y, et al. Immune cell infiltration-based characterization of triple-negative breast cancer predicts prognosis and chemotherapy response markers. Front Genet. 2021;12: 616469.
He Y, et al. Classification of triple-negative breast cancers based on Immunogenomic profiling. J Exp Clin Cancer Res. 2018;37(1):327.
Emens LA, et al. The tumor microenvironment (TME) and atezolizumab + nab-paclitaxel (A+nP) activity in metastatic triple-negative breast cancer (mTNBC): IMpassion130. J Clin Oncol. 2021;39(15):1006–1006.
Romero-Cordoba S, et al. Decoding immune heterogeneity of triple negative breast cancer and its association with systemic inflammation. Cancers (Basel). 2019;11(7):911.
Park JH, Ahn JH, Kim SB. How shall we treat early triple-negative breast cancer (TNBC): from the current standard to upcoming immuno-molecular strategies. ESMO Open. 2018;3(1): e000357.
Li CH, et al. Current treatment landscape for patients with locally recurrent inoperable or metastatic triple-negative breast cancer: a systematic literature review. Breast Cancer Res. 2019;21(1):143.
Cardoso F, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-updagger. Ann Oncol. 2019;30(8):1194–220.
Cardoso F, et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4)dagger. Ann Oncol. 2018;29(8):1634–57.
Fitzpatrick A, Tutt A. Controversial issues in the neoadjuvant treatment of triple-negative breast cancer. Ther Adv Med Oncol. 2019;11:1758835919882581.
Lebert JM, et al. Advances in the systemic treatment of triple-negative breast cancer. Curr Oncol. 2018;25(Suppl 1):S142–50.
von Minckwitz G, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804.
Bardia A, et al. Sacituzumab Govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741–51.
Kurani H, et al. DOT1L is a novel cancer stem cell target for triple-negative breast cancer. Clin Cancer Res. 2022;28(9):1948–65.
Sharma M, et al. Simultaneously targeting cancer-associated fibroblasts and angiogenic vessel as a treatment for TNBC. J Exp Med 2021;218(4):p. e20200712
Yang SJ, et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/beta-catenin (cyclin D1) axis. Cell Death Dis. 2021;12(5):420.
Xing L, et al. LncRNA HAND2-AS1 suppressed the growth of triple negative breast cancer via reducing secretion of MSCs derived exosomal miR-106a-5p. Aging (Albany NY). 2020;13(1):424–36.
Sulaiman A, et al. Targeting hypoxia sensitizes TNBC to cisplatin and promotes inhibition of both bulk and cancer stem cells. Int J Mol Sci. 2020;21(16):5788.
Chou Y-T, et al. Targeting triple-negative breast cancer with an aptamer-functionalized nanoformulation: a synergistic treatment that combines photodynamic and bioreductive therapies. J Nanobiotechnol. 2021;19(1):89.
Carroll CP, et al. Targeting hypoxia regulated sodium driven bicarbonate transporters reduces triple negative breast cancer metastasis. Neoplasia. 2022;25:41–52.
Mohammed MEA, Elhassan NM. Cytoskeletal and extracellular matrix proteins as markers for metastatic triple negative breast cancer. J Int Med Res. 2019;47(11):5767–76.
Phillips L, Gill AJ, Baxter RC. Novel prognostic markers in triple-negative breast cancer discovered by MALDI-mass spectrometry imaging. Front Oncol. 2019;9:379.
Wang RX, et al. Predictive and prognostic value of Matrix metalloproteinase (MMP)-9 in neoadjuvant chemotherapy for triple-negative breast cancer patients. BMC Cancer. 2018;18(1):909.
Mehanna J, et al. Triple-negative breast cancer: current perspective on the evolving therapeutic landscape. Int J Womens Health. 2019;11:431–7.
Pantelidou C, et al. PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov. 2019;9(6):722–37.
Li T, et al. Ribociclib (LEE011) suppresses cell proliferation and induces apoptosis of MDA-MB-231 by inhibiting CDK4/6-cyclin D-Rb-E2F pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):4001–11.
Hwang S-Y, Park S, Kwon Y. Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol Ther. 2019;199:30–57.
Mohamed RF, et al. Does bevacizumab carry a hope for metastatic triple-negative breast cancer in the era of immunotherapy? Anticancer Drugs. 2022;33(1):e604–e609.
Diamond JR, et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res Treat. 2020;184(1):53–62.
López-Nieva P, et al. More insights on the Use of γ-secretase inhibitors in cancer treatment. Oncologist. 2021;26(2):e298–305.
Bravaccini S, Maltoni R. Trop-2 therapy in metastatic triple-negative breast cancer in Italy: clinical opportunity and regulatory pitfalls. J Pers Med. 2021;11(11):1211.
Bardia A, et al. Sacituzumab Govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529–41.
Vahdat LT, et al. Glembatumumab vedotin for patients with metastatic, gpNMB overexpressing, triple-negative breast cancer (“METRIC”): a randomized multicenter study. NPJ Breast Cancer. 2021;7(1):57.
Yang S, Wei W, Zhao Q. B7–H3, a checkpoint molecule, as a target for cancer immunotherapy. Int J Biol Sci. 2020;16(11):1767–73.
Scribner JA, et al. Preclinical development of MGC018, a Duocarmycin-based antibody-drug conjugate targeting B7–H3 for solid cancer. Mol Cancer Ther. 2020;19(11):2235–44.
Bayerlova M, et al. Ror2 signaling and its relevance in breast cancer progression. Front Oncol. 2017;7:135.
Lin CW, et al. A new immunochemical strategy for triple-negative breast cancer therapy. Sci Rep. 2021;11(1):14875.
Bai X, et al. Immunotherapy for triple-negative breast cancer: a molecular insight into the microenvironment, treatment, and resistance. J Natl Cancer Center. 2021;1(3):75–87.
Barroso-Sousa R, et al. Nivolumab in combination with cabozantinib for metastatic triple-negative breast cancer: a phase II and biomarker study. NPJ Breast Cancer. 2021;7(1):110.
Loibl S, et al. Durvalumab improves long-term outcome in TNBC: results from the phase II randomized GeparNUEVO study investigating neodjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). J Clin Oncol. 2021;39(15):506–506.
Gandhi S, et al. Phase IIa study of alpha-DC1 vaccine against HER2/HER3, chemokine modulation regimen, and pembrolizumab in patients with asymptomatic brain metastasis from triple negative or HER2+ breast cancer. J Immunother Cancer 2020;8 (Suppl_3), A196-A197
Overman MJ, et al. Safety, efficacy and pharmacodynamics (PD) of MEDI9447 (oleclumab) alone or in combination with durvalumab in advanced colorectal cancer (CRC) or pancreatic cancer (panc). J Clin Oncol. 2018;36(15):4123–4123.
Telli ML, et al. Intratumoral plasmid IL12 expands CD8<sup>+</sup> T cells and induces a CXCR3 gene signature in triple-negative breast tumors that Sensitizes patients to anti–PD-1 therapy. Clin Cancer Res. 2021;27(9):2481–93.
Fang WB, et al. Expression of CCL2/CCR2 signaling proteins in breast carcinoma cells is associated with invasive progression. Sci Rep. 2021;11(1):8708.
Bu MT, et al. The roles of TGF-β and VEGF pathways in the suppression of antitumor immunity in melanoma and other solid tumors. Pharmacol Ther. 2022;240: 108211.
Raja J, et al. Oncolytic virus immunotherapy: future prospects for oncology. J Immunother Cancer. 2018;6(1):140.
Dees S, et al. Emerging CAR-T cell therapy for the treatment of triple-negative breast cancer. Mol Cancer Ther. 2020;19(12):2409–21.
Author information
Authors and Affiliations
Contributions
Writing original draft, prepare tables and figures: HMN; writing, prepare table and figure for section ADC and immunotherapies: WP; review and editing: HMN, WP, MO, and LW; conceptualization, funding acquisition and supervision: LW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Nguyen, HM., Paulishak, W., Oladejo, M. et al. Dynamic tumor microenvironment, molecular heterogeneity, and distinct immunologic portrait of triple-negative breast cancer: an impact on classification and treatment approaches. Breast Cancer 30, 167–186 (2023). https://doi.org/10.1007/s12282-022-01415-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-022-01415-4