Abstract
Background
Breast cancer is the most common malignancy among women worldwide. As survival rates increase, breast reconstruction and quality of life gain importance. Of all women undergoing breast reconstruction, approximately, 70% opt for silicone implants and 50% of those develop capsular contracture, the most prevalent long-term complication. The collagenase of the bacterium Clostridium histolyticum (CCH) showed promising results in the therapy of capsule contracture; however, its influence on residual cancer cells is unknown. The aim of this study was to investigate whether CCH-treatment negatively impacts breast cancer cells in vitro and in vivo.
Methods
MDA-MB-231 and MCF-7 cells were used in this study. In vitro, we tested the influence of CCH on proliferation, wound healing, migration and cell cycle by MTT-assay, scratch-assay, transwell-migration-assay, and flow cytometry.
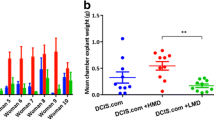
In vivo, solid tumors were induced in immune-deficient mice. CCH was injected into the tumors and tumor growth and metastasis formation was monitored by caliper measurement, in vivo bioluminescence imaging and histology. Gene expression analysis was performed by microarray including 27,190 genes.
Results
CCH-incubation led to a dose-dependent reduction in proliferation for both cell lines, while wound healing was reduced only in MDA-MB-231 cells. No morphological alterations were monitored in cell cycle or apoptosis. In vivo, bioluminescence imaging and histology did not show any evidence of metastasis. Although CCH led to changes in gene expression of breast cancer cells, no relevant alterations in metastasis-related genes were monitored.
Conclusion
CCH has no impact on tumor growth or metastasis formation in vitro and in vivo. This paves the way for first clinical trials.







Similar content being viewed by others
References
Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther. 2019;11:151.
Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50(1):33.
Miller AM, Steiner CA, Barrett ML, Fingar KR, Elixhauser A. Breast reconstruction surgery for mastectomy in hospital inpatient and ambulatory settings, 2009–2014: statistical brief# 228. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville: Agency for Healthcare Research and Quality (US); 2017.
Pomahac B, Recht A, May JW, Hergrueter CA, Slavin SA. New trends in breast cancer management: is the era of immediate breast reconstruction changing? Ann Surg. 2006;244(2):282–8.
Wick G, Backovic A, Rabensteiner E, Plank N, Schwentner C, Sgonc R. The immunology of fibrosis: innate and adaptive responses. Trends Immunol. 2010;31(3):110–9.
Gurunluoglu R, Sacak B, Arton J. Outcomes analysis of patients undergoing autoaugmentation after breast implant removal. Plast Reconstr Surg. 2013;132(2):304–15.
Diehm YF, Hirche C, Berger MR, Heil J, Golatta M, Kotsougiani D, et al. The collagenase of the bacterium Clostridium histolyticum in the treatment of irradiation-induced capsular contracture. Aesthetic Plast Surg. 2019;43(3):836–44.
Fischer S, Diehm Y, Henzler T, Berger MR, Kolbenschlag J, Latz A, et al. Long-term effects of the collagenase of the bacterium Clostridium histolyticum for the treatment of capsular fibrosis after silicone implants. Aesthetic plast Surg. 2016;41:211–20.
Fischer S, Hirche C, Diehm Y, Nuutila K, Kiefer J, Gazyakan E, et al. Efficacy and safety of the collagenase of the bacterium Clostridium Histolyticum for the treatment of capsular contracture after silicone implants: ex-vivo study on human tissue. PLoS ONE. 2016;11(5):e0156428.
Fischer S, Hirsch T, Diehm Y, Kiefer J, Bueno EM, Kueckelhaus M, et al. The collagenase of the bacterium Clostridium histolyticum for the treatment of capsular fibrosis after silicone implants. Plast Reconstr Surg. 2015;136(5):981–9.
Holzer LA, Holzer G. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. New Engl J Med. 2009;361(26):2579 (author reply 2579–2580).
Tsambarlis P, Levine LA. Nonsurgical management of Peyronie’s disease. Nat Rev Urol. 2019;16(3):172–86.
Kovacheva M, Zepp M, Berger SM, Berger MR. Sustained conditional knockdown reveals intracellular bone sialoprotein as essential for breast cancer skeletal metastasis. Oncotarget. 2014;5(14):5510.
Benton G, Kleinman HK, George J, Arnaoutova I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int J Cancer. 2011;128(8):1751–7.
Wetterwald A, van der Pluijm G, Que I, Sijmons B, Buijs J, Karperien M, et al. Optical imaging of cancer metastasis to bone marrow: a mouse model of minimal residual disease. Am J Pathol. 2002;160(3):1143–53.
Shi L, Jones WD, Jensen RV, Harris SC, Perkins RG, Goodsaid FM, et al. The balance of reproducibility, sensitivity, and specificity of lists of differentially expressed genes in microarray studies. BMC Bioinform. 2008;9(Suppl 9):S10.
Kittleson MM, Minhas KM, Irizarry RA, Ye SQ, Edness G, Breton E, et al. Gene expression in giant cell myocarditis: altered expression of immune response genes. Int J Cardiol. 2005;102(2):333–40.
Zhao B, Erwin A, Xue B. How many differentially expressed genes: a perspective from the comparison of genotypic and phenotypic distances. Genomics. 2018;110(1):67–73.
Gordon NH, Siminoff LA. Measuring quality of life of long-term breast cancer survivors: the long term quality of life–breast cancer (LTQOL-BC) scale. J Psychosoc Oncol. 2010;28(6):589–609.
Diehm YF, Jost Y, Kotsougiani-Fischer D, Haug V, Splinter M, Häring P, et al. The treatment of capsular contracture around breast implants induced by fractionated irradiation: the collagenase of the bacterium Clostridium Histolyticum as a novel therapeutic approach. Aesthetic Plast Surg. 2020;45:1273–81.
Syed F, Thomas AN, Singh S, Kolluru V, Emeigh Hart SG, Bayat A. In vitro study of novel collagenase (XIAFLEX®) on Dupuytren’s disease fibroblasts displays unique drug related properties. PLoS ONE. 2012;7(2):e31430.
Balko JM, Cook RS, Vaught DB, Kuba MG, Miller TW, Bhola NE, et al. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nat Med. 2012;18(7):1052–9.
Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17(5):548–58.
Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–7.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–98.
De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123(10):2229–38.
Miyazaki K, Togo S, Okamoto R, Idiris A, Kumagai H, Miyagi Y. Collective cancer cell invasion in contact with fibroblasts through integrin-α5β1/fibronectin interaction in collagen matrix. Cancer Sci. 2020;111:4381–92.
Bachem MG, Schünemann M, Ramadani M, Siech M, Beger H, Buck A, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128(4):907–21.
Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264(1):169–84.
Bates RC, Mercurio AM. Tumor necrosis factor-α stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell. 2003;14(5):1790–800.
Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67.
Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24(6):389–99.
Wang JP, Hielscher A. Fibronectin: how its aberrant expression in tumors may improve therapeutic targeting. J Cancer. 2017;8(4):674.
Attieh Y, Clark AG, Grass C, Richon S, Pocard M, Mariani P, et al. Cancer-associated fibroblasts lead tumor invasion through integrin-β3–dependent fibronectin assembly. J Cell Biol. 2017;216(11):3509–20.
Erdogan B, Ao M, White LM, Means AL, Brewer BM, Yang L, et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J Cell Biol. 2017;216(11):3799–816.
Gopal S, Veracini L, Grall D, Butori C, Schaub S, Audebert S, et al. Fibronectin-guided migration of carcinoma collectives. Nat Commun. 2017;8(1):1–15.
Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83(3):249–89.
Satya-Prakash KL, Pathak S, Hsu TC, Olivé M, Cailleau R. Cytogenetic analysis on eight human breast tumor cell lines: high frequencies of 1q, 11q and HeLa-like marker chromosomes. Cancer Genet Cytogenet. 1981;3(1):61–73.
Lee AV, Oesterreich S, Davidson NE. MCF-7 cells—changing the course of breast cancer research and care for 45 years. J Nat Cancer Inst. 2015;107(7):djv073
Sussman BJ. Intervertebral discolysis with collagenase. J Natl Med Assoc. 1968;60(3):184–7.
Sussman BJ. Experimental intervertebral discolysis. A critique of collagenase and chymopapain applications. Clin Orthop Relat Res. 1971; 80:181–190.
Sussman BJ, Bromley JW, Gomez JC. Injection of collagenase in the treatment of herniated lumbar disk. Initial Clin Rep JAMA. 1981;245(7):730–2.
Sussman BJ, Mann M. Experimental intervertebral discolysis with collagenase. J Neurosurg. 1969;31(6):628–35.
Badalamente MA, Hurst LC. Enzyme injection as a nonoperative treatment for Dupuytren’s disease. Drug Deliv. 1996;3(1):35–40.
Gelbard MK, Walsh R, Kaufman JJ. Collagenase for Peyronie’s disease experimental studies. Urol Res. 1982;10(3):135–40.
Acknowledgements
None.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Methodology: YFD, KM, AMS, JT, MZ, MMG, and SF. Formal analysis and investigation: YFD, MRB, UK, and SF. Writing—original draft preparation: YFD, DKF, and SF. Writing—review and editing: all the authors. Supervision: MRB, UK, and SF.
Corresponding author
Ethics declarations
Conflict of interest
The authors received funding support through an Educational Research Grant from Endo Pharmaceuticals (Malvern, USA). This grant was used to purchase general lab supply, both breast cancer cell lines, assays and immune-deficient mice. None of the authors received a salary for this study or has any financial interest.
Statement on the welfare of animals
All applicable national and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Diehm, Y.F., Marstaller, K., Seckler, AM. et al. The collagenase of the bacterium Clostridium histolyticum does not favor metastasis of breast cancer. Breast Cancer 29, 599–609 (2022). https://doi.org/10.1007/s12282-022-01337-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-022-01337-1