Abstract
Purpose
Radiotherapy (RT) and endocrine therapy (ET) are standard treatment options after breast-conserving surgery (BCS) for ductal carcinoma in situ (DCIS). We investigated the national patterns of adjuvant therapy use after BCS for DCIS in Japan.
Methods
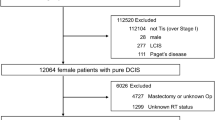
We obtained relevant data of patients diagnosed with DCIS undergoing surgery and treated with BCS between 2014 and 2016 from the Japanese Breast Cancer Registry database. The relationship between the clinicopathologic, institutional, and regional factors, and adjuvant treatment was examined using multivariable analyses.
Results
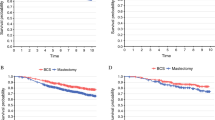
We identified 9516 patients who underwent BCS for DCIS. Overall, 23% received no adjuvant treatment, 71% received RT, 32% received ET, and 26% received combination therapy. The percentages of patients who received ET and combination therapy in 2016 were significantly lower [odds ratio (OR): 0.71, 0.77, respectively] than in 2014. The proportion of RT was low among young or elderly patients (OR: 0.75, 0.44, respectively) and in non-certified facilities (OR: 0.56). The proportion of ET was high in non-certified facilities (OR: 1.58) and among patients with positive margins (OR: 1.62). Combination therapy was higher among patients with positive margins (OR: 1.53).
Conclusions
Our study found a distinct adjuvant treatment pattern after BCS for DCIS depending on clinicopathologic factors, year, age, which indicate that physicians provide individualized treatment according to the background of the patients and the biology of DCIS. The facilities and regions remain significant factors of influencing adjuvant treatment pattern.


Similar content being viewed by others
Abbreviations
- BCS:
-
Breast-conserving surgery
- DCIS:
-
Ductal carcinoma in situ
- ET:
-
Endocrine therapy
- IBTR:
-
Ipsilateral breast tumor recurrence
- JBCS:
-
Japanese Breast Cancer Society
- RT:
-
Radiotherapy
References
Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375:1438–47.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin. 2015;65:5–29.
Virnig BA, Shamliyan T, Tuttle TM, Kane RL, Wilt TJ. Diagnosis and management of ductal carcinoma in situ (DCIS). Evid Rep Technol Assess. 2009;185:1–549.
Kubo M, Kumamaru H, Isozumi U, Miyashita M, Nagahashi M, Kadoya T, et al. Annual report of the Japanese Breast Cancer Society registry for 2016. Breast Cancer. 2020;27:511–8. https://doi.org/10.1007/s12282-020-01081-4.
Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–8.
Vidali C, Caffo O, Aristei C, Bertoni F, Bonetta A, Guenzi M, et al. Conservative treatment of breast ductal carcinoma in situ: results of an Italian multi-institutional retrospective study. Radiat Oncol. 2012;7:177. https://doi.org/10.1186/1748-717X-7-177.
Shaitelman SF, Wilkinson Ben J, Kestin LL, Ye H, Goldstein NS, Martinez AA, et al. Long-term outcome in patients with ductal carcinoma in situ treated with breast-conserving therapy: implications for optimal follow-up strategies. Int J Radiat Oncol Biol Phys. 2012;83:e305–12. https://doi.org/10.1016/j.ijrobp.2011.12.092.
Boughey JC, Gonzalez RJ, Bonner E, Kuerer HM. Current treatment and clinical trial developments for ductal carcinoma in situ of the breast. Oncologist. 2007;12:1276–87.
Wapnir IL, Dignam JJ, Fisher B, Mamounas EP, Anderson SJ, Julian TB, et al. Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst. 2011;103:478–88.
Davidson N, Gelber R, Piccart M, Pruneri G, Pritchard K, Ravdin P, et al. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Inst Monogr. 2010;41:162–77.
Goodwin A, Parker S, Ghersi D, Wilcken N. Post-operative radiotherapy for ductal carcinoma in situ of the breast. Cochrane Database Syst Rev. 2013. http://doi.wiley.com/10.1002/14651858.CD000563.pub7
Sagara Y, Freedman RA, Vaz-Luis I, Mallory MA, Wong SM, Aydogan F, et al. Patient prognostic score and associations with survival improvement offered by radiotherapy after breast-conserving surgery for ductal carcinoma in situ: a population-based longitudinal cohort study. J Clin Oncol. 2016;34:1190–6.
Sagara Y, Freedman RA, Wong SM, Aydogan F, Nguyen A, Barry WT, et al. Trends in adjuvant therapies after breast-conserving surgery for hormone receptor-positive ductal carcinoma in situ : findings from the National Cancer Database, 2004–2013. Breast Cancer Res Treat. 2017;166(2):583–92.
Kurebayashi J, Miyoshi Y, Ishikawa T, Saji S, Sugie T, Suzuki T, et al. Clinicopathological characteristics of breast cancer and trends in the management of breast cancer patients in Japan: based on the Breast Cancer Registry of the Japanese Breast Cancer Society between 2004 and 2011. Breast Cancer. 2015;22:235–44. https://doi.org/10.1007/s12282-015-0599-6.
Greene F. TNM classification of malignant tumours. 6th ed. New York: Wiley; 2002. p. 131–41.
The Japanese Breast Cancer Society, editor. General rules for clinical and pathological recording of breast cancer. 17th ed. Tokyo: Kanehara Shuppan; 2012.
Lakhani S, Ellis I, Schnitt S, Tan P, van de Vijver M. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press; 2012.
Silverstein MJ, Lagios MD, Craig PH, Waisman JR, Lewinsky BS, Colburn WJ, et al. A prognostic index for ductal carcinoma in situ of the breast. Cancer. 1996;77(11):2267–74. https://doi.org/10.1002/(SICI)1097-0142(19960601)77:11%3c2267::AID-CNCR13%3e3.0.CO;2-V (PMID: 8635094).
Silverstein MJ. The University of Southern California/Van Nuys prognostic index for ductal carcinoma in situ of the breast. Am J Surg. 2003;186(4):337–43. https://doi.org/10.1016/s0002-9610(03)00265-4 (PMID: 14553846).
The Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer. Tokyo: Kanehara Shuppan; 2013.
The Japanese Breast Cancer Society Clinical Practice Guidelines for Breast Cancer. Tokyo: Kanehara Shuppan; 2015.
Narod SA, Iqbal J, Giannakeas V, Sopik V, Sun P. Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. 2015;1:888–96.
Hughes LL, Wang M, Page DL, Gray R, Solin LJ, Davidson NE, et al. Local excision alone without irradiation for ductal carcinoma in situ of the breast: a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2009;27:5319–24.
Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–30.
Lambertini M, Kroman N, Ameye L, Cordoba O, Pinto A, Benedetti G, et al. Long-term safety of pregnancy following breast cancer according to estrogen receptor status. JNCI J Natl Cancer Inst. 2017;110:426–9. https://doi.org/10.1093/jnci/djx206.
Iqbal J, Amir E, Rochon PA, Giannakeas V, Sun P, Narod SA. Association of the timing of pregnancy with survival in women with breast cancer. JAMA Oncol. 2017;3:659–65. https://doi.org/10.1001/jamaoncol.2017.0248.
Pregnancy Outcome and Safety of Interrupting Therapy for Women With Endocrine Responsive Breast Cancer (POSITIVE). https://clinicaltrials.gov/ct2/show/NCT02308085 Accessed July 2020
Acknowledgements
This report was supported in part by the National Cancer Center Research and Development fund (26-A-4) from the Ministry of Health, Labour and Welfare and the Practical Research for Innovative Cancer Control (19ck0106307h0002) from the Japan Agency for Medical Research and Development, AMED. The grammatical assistance was performed by professional editors at Editage, a division of Cactus Communications (www.editage.com).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hiraku Kumamaru and Hiroaki Miyata are affiliated with the department of Healthcare Quality Assessment at the university of Tokyo. The department is a social collaboration department supported by National Clinical Database, Johnson & Johnson K.K., and Nipro Corporation. Naoki Niikura has received grants and personal fees from Chugai, Pfizer, Eisai, Daiichi Sankyo, MSD, grants from Novartis, Eisai, AstraZeneka, Kyowa Hakko Kirin, Nippon Mediphysics, Takeda, Taiho, Nippon Kayaku, Eli Lilly. Hitoshi Tsuda has received grants from Taiho, Goryo Chemical, personal fees and other from Chugai, other from Takeda, Eli Lilly. Yutaka Yamamoto has received grants and personal fees from Chugai, Kyowa Hakko Kirin, Eli Lilly, Nihon Kayaku, personal fees from Taiho, Daiichi Sankyo, AstraZeneca, Novartis, Eisai, Takeda, Pfizer. Naoki Hayashi has received personal fees from Chugai, Novartis, Pfizer, Daiichi Sankyo, AstraZeneca, Genomic Health inc. Minoru Miyashita has received personal fees from Chugai, Eli Lilly, Eisai, AstraZeneca, Pfizer, Taiho, Daiichi Sankyo, non-financial support from Kyowa Hakko Kirin. Kenjiro Aogi has received personal fees as honoraria from Chugai, Eisai, AstraZeneca, Taiho, Novartis, Daiichi Sankyo, Mochida, Ono, Eli Lilly, and his institution received research funds from Chuai, Eisai, Takeda. Shigehira Saji has received grants and personal fees from Chugai, Kyowa Hakko Kirin, Eli Lilly, AstraZeneca, Novartis, Eisai, Takeda, personal fees from Pfizer, MSD, Daiichi Sankyo, grants from Taiho. Masakazu Toi has received grants and personal fees from Chugai, Takeda, Pfizer, Kyowa Hakko Kirin, C & C Res Lab, Taiho, Eisai, Daiichi Sankyo, AstraZeneca, grants from JBCRG association, Astellas, personal fees from Eli Lilly, MSD, Genomic Health, Novartis, Konica Minolta, outside the submitted work; and Board of directors; JBCRG association, Organization for Oncology and Translational Research, Kyoto Breast cancer Research Network. The other authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Yotsumoto, D., Sagara, Y., Kumamaru, H. et al. Trends in adjuvant therapy after breast-conserving surgery for ductal carcinoma in situ of breast: a retrospective cohort study using the National Breast Cancer Registry of Japan. Breast Cancer 29, 1–8 (2022). https://doi.org/10.1007/s12282-021-01307-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-021-01307-z