Abstract
Purpose
Second-line endocrine therapy (ET) for estrogen receptor (ER)-positive and human epidermal growth factor 2 (HER2)-negative metastatic breast cancer (MBC) is offered based on the response to first-line ET. However, no clinical trials have evaluated the efficacy and safety of secondary ETs in patients with poor responses to initial ET. This study evaluated the efficacy of second-line ET in ER-positive and HER2-negative postmenopausal MBC patients with low or very low sensitivity to initial ET.
Methods
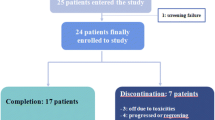
This multicenter prospective observational cohort study evaluated the response of 49 patients to second-line ETs in postmenopausal MBC patients with low or very low sensitivity to initial ET. The primary endpoint was the clinical benefit rate (CBR) for 24 weeks.
Results
Of the 49 patients assessed, 40 (82%) received fulvestrant in the second line, 5 (10%) received selective estrogen receptor modulators, 3 (6%) received aromatase inhibitors (AIs) alone, and 1 received everolimus with a steroidal AI. The overall CBR was 44.9% [90% confidence interval (CI): 34.6–57.6, p = 0.009]; CBR demonstrated similar significance across the progesterone receptor-positive (n = 39, 51.3%, 90% CI: 39.6–65.2, p = 0.002), very low sensitivity (n = 17, 58.8%, 90% CI: 42.0–78.8, p = 0.003), and non-visceral metastases (n = 25, 48.0%, 90% CI: 34.1–65.9, p = 0.018) groups. The median progression-free survival was 7.1 months (95% CI: 5.6–10.6).
Conclusion
Second-line ET might be a viable treatment option for postmenopausal patients with MBC with low and very low sensitivity to initial ET. Future studies based on larger and independent cohorts are needed to validate these findings.



Similar content being viewed by others
References
Yamamoto N, Watanabe T, Katsumata N, Omuro Y, Ando M, Fukuda H, et al. Construction and validation of a practical prognostic index for patients with metastatic breast cancer. J Clin Oncol. 1998;16(7):2401–8. https://doi.org/10.1200/JCO.1998.16.7.2401.
Watanabe T. Evidence produced in Japan: tegafur-based preparations for postoperative chemotherapy in breast cancer. Breast Cancer. 2013;20(4):302–9. https://doi.org/10.1007/s12282-013-0451-9.
Chlebowski TR. Strategies to overcome endocrine therapy resistance in hormone receptor-positive advanced breast cancer. Clin Invest. 2014;4(1):19–33.
Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339(14):974–84. https://doi.org/10.1056/NEJM199810013391407.
Chlebowski RT. Changing concepts of hormone receptor-positive advanced breast cancer therapy. Clin Breast Cancer. 2013;13(3):159–66. https://doi.org/10.1016/j.clbc.2012.11.002.
Barrios C, Forbes JF, Jonat W, Conte P, Gradishar W, Buzdar A, et al. The sequential use of endocrine treatment for advanced breast cancer: where are we? Ann Oncol. 2012;23(6):1378–86. https://doi.org/10.1093/annonc/mdr593.
Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2)dagger. Ann Oncol. 2014;25(10):1871–88. https://doi.org/10.1093/annonc/mdu385.
Iwamoto T, Taira N, Fujisawa T, Araki K, Sakamaki K, Sangai T, et al. Hormonal therapy resistant estrogen-receptor positive metastatic breast cancer cohort (HORSE-BC) study: current status of treatment selection in Japan. Acta Med Okayama. 2018;72(4):369–74. https://doi.org/10.18926/AMO/56172.
Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106(Pt 4):1377–88.
DeFriend DJ, Anderson E, Bell J, Wilks DP, West CM, Mansel RE, et al. Effects of 4-hydroxytamoxifen and a novel pure antioestrogen (ICI 182780) on the clonogenic growth of human breast cancer cells in vitro. Br J Cancer. 1994;70(2):204–11.
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–9. https://doi.org/10.1056/NEJMoa1109653.
Piccart M, Hortobagyi GN, Campone M, Pritchard KI, Lebrun F, Ito Y, et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: overall survival results from BOLERO-2dagger. Ann Oncol. 2014;25(12):2357–62. https://doi.org/10.1093/annonc/mdu456.
Robertson JF, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet. 2016;388(10063):2997–3005. https://doi.org/10.1016/S0140-6736(16)32389-3.
Taira N, Fujisawa T, Araki T, Iwamoto T, Sakamaki K, Takahashi M et al (2016) Cohort study of secondary endocrine therapy in metastatic breast cancer with a poor response to initial endocrine therapy. J Clin Trials 6(2)
Robertson JF, Howell A, Gorbunova VA, Watanabe T, Pienkowski T, Lichinitser MR. Sensitivity to further endocrine therapy is retained following progression on first-line fulvestrant. Breast Cancer Res Treat. 2005;92(2):169–74. https://doi.org/10.1007/s10549-004-4776-0.
Toy W, Shen Y, Won H, Green B, Sakr RA, Will M, et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet. 2013;45(12):1439–45. https://doi.org/10.1038/ng.2822.
Ellis MJ, Llombart-Cussac A, Feltl D, Dewar JA, Jasiowka M, Hewson N, et al. Fulvestrant 500 mg versus anastrozole 1 mg for the first-line treatment of advanced breast cancer: overall survival analysis from the phase II FIRST study. J Clin Oncol. 2015;33(32):3781–7. https://doi.org/10.1200/JCO.2015.61.5831.
Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, et al. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell Rep. 2013;4(6):1116–30. https://doi.org/10.1016/j.celrep.2013.08.022.
Fribbens C, O'Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M, et al. Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2016;34(25):2961–8. https://doi.org/10.1200/JCO.2016.67.3061.
Ravdin PM, Green S, Dorr TM, McGuire WL, Fabian C, Pugh RP, et al. Prognostic significance of progesterone receptor levels in estrogen receptor-positive patients with metastatic breast cancer treated with tamoxifen: results of a prospective Southwest Oncology Group study. J Clin Oncol. 1992;10(8):1284–91. https://doi.org/10.1200/JCO.1992.10.8.1284.
Prat A, Cheang MC, Martin M, Parker JS, Carrasco E, Caballero R, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31(2):203–9. https://doi.org/10.1200/JCO.2012.43.4134.
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35. https://doi.org/10.1016/S1470-2045(14)71159-3.
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–36. https://doi.org/10.1056/NEJMoa1607303.
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39. https://doi.org/10.1016/S1470-2045(15)00613-0.
Acknowledgements
This study was conducted as a research support project of the General Incorporated Association of Comprehensive Support Project for Oncological Research of Breast Cancer (CSPOR-BC). We would like to thank Editage (www.editage.com) for English language editing.
Funding
This study was funded by AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TI, TF, TShien, KA, KS, TSangai, ST, RN, TA, HM, and NT declare no conflict of interest. YK received an honorarium from Eizai, Chugai, Novartis, Taiho, Pfizer, and Eli Lilly. MT received an honorarium from AstraZeneca, Eli Lilly, Eizai, and Pfizer, and research funding from Eizai, Kyowa Hakko Kirin, Taiho, and Nippon kayaku.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12282_2020_1095_MOESM1_ESM.tif
Supplementary file 1 Supplementary Fig. 1 Kaplan–Meier curves for time to chemotherapy. (A) All patients (N = 49). (B) Fulvestrant (n = 40), CI confidence interval (TIF 55 kb)
About this article
Cite this article
Iwamoto, T., Fujisawa, T., Shien, T. et al. The efficacy of sequential second-line endocrine therapies (ETs) in postmenopausal estrogen receptor-positive and HER2-negative metastatic breast cancer patients with lower sensitivity to initial ETs. Breast Cancer 27, 973–981 (2020). https://doi.org/10.1007/s12282-020-01095-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-020-01095-y