Abstract
Purpose
To investigate the safety, pharmacokinetics, and efficacy of trastuzumab emtansine (T-DM1) in combination with pertuzumab in Japanese patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer.
Patients and methods
Patients with HER2-positive advanced or recurrent breast cancer who had received trastuzumab and chemotherapy-containing therapies were eligible. Patients received T-DM1 (3.6 mg/kg) with full-dose pertuzumab (a loading dose of 840 mg and then 420 mg) intravenously every 3 weeks. This study was registered at the Japan Pharmaceutical Information Center-Clinical Trials Information (JapicCTI-101234).
Results
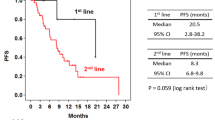
Six patients enrolled in this study. The median duration of treatment was 11 (range 1–32) cycles. The most common treatment-emergent adverse event (TEAE) for any grade was diarrhea. Grade 3 or greater TEAEs included aspartate aminotransferase increased, left ventricular ejection fraction (LVEF) decreased, and neutrophil count decreased. The dose-limiting toxicity of grade 3 LVEF decreased was observed in one patient during cycle 1; however, it resolved within 30 days. The pharmacokinetic parameters of T-DM1 and pertuzumab were not affected by co-administration of the drugs. The best overall response included a partial response (PR) in 3 patients (50%) and stable disease (SD) in 2 patients (33%).
Conclusions
The combination of T-DM1 and pertuzumab was tolerated and showed exploratory efficacy in Japanese patients with HER2-positive metastatic breast cancer.



Similar content being viewed by others
References
Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–91.
Diéras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–42.
Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:689–99.
Krop IE, Kim SB, Martin AG, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017;18:743–54.
Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–19.
Swain SM, Kim SB, Cortes J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–71.
Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34.
Phillips GD, Fields CT, Li G, et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res. 2014;20:456–68.
Martin M, Fumoleau P, Dewar JA, et al. Trastuzumab emtansine (T-DM1) plus docetaxel with or without pertuzumab in patients with HER2-positive locally advanced or metastatic breast cancer: results from a phase Ib/IIa study. Ann Oncol. 2016;27:1249–56.
Perez EA, Barrios C, Eiermann W, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35:141–8.
Perez EA, Barrios CH, Eiermann W, et al. Phase III, randomized study of first-line trastuzumab emtansine (T-DM1) ± pertuzumab (P) vs. trastuzumab + taxane (HT) treatment of HER2-positive MBC: final overall survival (OS) and safety from MARIANNE. J Clin Oncol. 2017;35(suppl abstr 1003).
Yamamoto H, Ando M, Aogi K, et al. Phase I and pharmacokinetic study of trastuzumab emtansine in Japanese patients with HER2-positive metastatic breast cancer. Jpn J Clin Oncol. 2015;45:12–8.
Yamamoto N, Yamada Y, Fujiwara Y, et al. Phase I and pharmacokinetic study of HER2-targeted rhuMAb 2C4 (Pertuzumab, RO4368451) in Japanese patients with solid tumors. Jpn J Clin Oncol. 2009;39:260–6.
Hurvitz SA, Martin M, Symmans WF, et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018;19:115–26.
Acknowledgements
We would like to thank the patients who participated in this trial and their families, the investigators, and all staffs who supported this trial.
Funding
This study was sponsored by Chugai Pharmaceutical Co., Ltd. and F. Hoffmann-La Roche Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
KT and JH declare no conflicts of interest. EN has received honoraria from Chugai and Novartis. MH has received honoraria from Chugai, AstraZeneca, Eli Lilly, Pfizer, Eisai, Daiichi-Sankyo, and Novartis. NS has received remuneration from Chugai, AstraZeneca, Eisai, Pfizer, and Taiho. KK and KM are employees of Chugai. HI has received grants from Chugai, AstraZeneca, Bayer, Eli Lilly, GSK, Kyowa Hakko Kirin, MSD, Novartis, and Pfizer; honoraria from Chugai, AstraZeneca, Eisai, and Pfizer. YF has received grants from Chugai and Takeda; honoraria from Chugai, Astra Zeneca, Daiichi-Sankyo, Eisai, Eli Lilly, Novartis, and Taiho.
About this article
Cite this article
Noguchi, E., Tamura, K., Hattori, M. et al. Trastuzumab emtansine plus pertuzumab in Japanese patients with HER2-positive metastatic breast cancer: a phase Ib study. Breast Cancer 26, 39–46 (2019). https://doi.org/10.1007/s12282-018-0887-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-018-0887-z