Abstract
Background
Ixabepilone is now a Food and Drug Administration-approved therapeutic option for patients with metastatic breast cancer (MBC) whose disease has progressed despite prior anthracycline and taxane therapy. We conducted a systematic review and meta-analysis to systematically evaluate the efficacy and safety of ixabepilone for treating metastatic breast cancer.
Methods
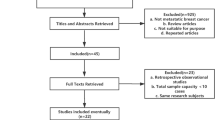
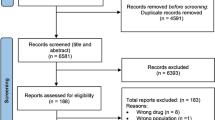
A systematic review and meta-analysis were conducted. Randomized controlled studies applying ixabepilone for treating MBC were included. The primary outcome was Overall Survival (OS). The authors of primary articles were contacted and methodological quality was evaluated. Subgroups were drawn based on intervention measures; heterogeneity and bias were discussed.
Results
Eight studies with 5247 patients were included. Compared with a weekly schedule, a triweekly schedule of ixabepilone was better at improving overall response rate (ORR), while there were no differences in improving OS and progression-free survival (PFS). Ixabepilone plus capecitabine was superior to capecitabine monotherapy in improving OS, PFS and ORR. Paclitaxel was more effective than ixabepilone in terms of OS and PFS. There was no difference in the improvement of ORR, clinical benefit rate (CBR) and disease control rate (DCR) between ixabepilone and eribulin.
Conclusions
Current evidence suggests that a triweekly schedule of ixabepilone is more effective than weekly dosing in improving ORR. Use of ixabepilone in combination with capecitabine possesses superior clinical efficacy to the use of capecitabine alone. Paclitaxel was more effective than ixabepilone in terms of OS and PFS. The efficacy and safety between ixabepilone and eribulin were identical.

Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reducecancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–50.
Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–93.
Fumoleau P, Largillier R, Clippe C, et al. Multicentre, phase II study evaluating capecitabine monotherapy in patients with anthracycline- and taxane-pretreated metastatic breast cancer. Eur J Cancer. 2004;40:536–42.
Altmann KH. Epothilone B and its analogs—a new family of anticancer agents. Mini Rev Med Chem. 2003;3:149–58.
Lee F, Lewin A, Wen M, Ryseck R, Fargnoli J, Poruchynsky M, Fojo T, Mudenda B, Rugo H. Antiangiogenic (AG) synergywithixabepilone (ixa): translation of preclinical studies tothe clinical setting. Cancer Res. 2009;69:20.
Lee FY, Covello KL, Castaneda S, Hawken DR, Kan D, LewinA Wen ML, Ryseck RP, Fairchild CR, Fargnoli J, et al. Synergistic antitumor efficacy of ixabepilone (BMS-247550) plus bevacizumab in multiple in vivo tumor models. Clin Cancer Res. 2008;14:8123–31.
Paradiso A, Mangia A, Chiriatti A, Tommasi S, Zito A, et al. Biomarkers predictive for clinical efficacy of taxol-based chemotherapy in advanced breast cancer. Ann Oncol. 2005;16(Suppl 4):iv14–9.
Seve P, Isaac S, Tredan O, Souquet PJ, Pacheco Y, et al. Expression of class III {beta}-tubulin is predictive of patient outcome in patients with nonsmall cell lung cancer receiving vinorelbine-based chemotherapy. Clinl Cancer Res. 2005;11:5481–6.
Andreopoulou E, Muggia F. Pharmacodynamics of tubulin and tubulinbinding agents: extending their potential beyond taxanes. Clin Breast Cancer. 2008;8(Suppl 2):S54–60.
Fountzilas G, Kotoula V, Pectasides D, Kouvatseas G, Timotheadou E, et al. Ixabepilone administered weekly or every three weeks in HER2-negative metastatic breast cancer patients; a randomized non-comparative phase II trial. PLoS One. 2013;8:e69256.
Rugo HS, Campone M, Amadori D, Wardley AM, Aldrighetti D, Conte PF, Liu D, Mudenda B, McHenry MB. Pivot XB (2013) A randomized, phase II, three-arm study of two schedules of ixabepilone or paclitaxel plus bevacizumab as first-line therapy for metastatic breast cancer. Breast Cancer Res Treat. 2013;139:411–9.
Smith JW, Vukelja SJ, Rabe AC, et al. Phase II randomized trial of weekly and every-3-week ixabepilone in metastatic breast cancer patients. Breast Cancer Res Treat. 2013;142:381–8.
Sparano JA, Vrdoljak E, Rixe O, Xu B, et al. Randomized phase III trial of ixabepilone plus capecitabine versus capecitabine in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2010;28:3256–62.
Thomas ES, Gomez HL, Li RK, et al. Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and taxane treatment. J Clin Oncol. 2007;25:5210–7.
Vahdat LT, Garcia AA, Vogel C, et al. Eribulin mesylate versus ixabepilone in patients with metastatic breast cancer: a randomized Phase II study comparing the incidence of peripheral neuropathy. Breast Cancer Res Treat. 2013;140:341–51.
Vahdat LT, Vrdoljak E, Gómezc H, et al. Efficacy and safety of ixabepilone plus capecitabine in elderly patients with anthracycline- and taxane-pretreated metastatic breast cancer. J Geriatr Oncol. 2013;4:346–52.
Rugo HS, Barry WT, Aspitia AM, et al. Randomized phase III trial of paclitaxel once per week compared with nanoparticle albumin-bound nab-paclitaxel once per week or ixabepilone with bevacizumab as first-line chemotherapy for locally recurrent or metastatic breast cancer: CALGB40502/NCCTG N063H (Alliance). J Clin Oncol. 2015;33:2361–9.
Myers Squibb Company. IXEMPRA® (ixabepilone) prescribing information. Bristol-Myers Squibb Company, Princeton, NJ.
Moulder S, Li H, Wang M, Gradishar WJ, Perez EA, Sparano JA, Pins M, Yang X, Sledge GW. A phase II trial of trastuzumab plus weekly ixabepilone and carboplatin in patients with HER2-positive metastatic breast cancer: an Eastern Cooperative Oncology Group Trial. Breast Cancer Res Treat. 2010;119:663–71.
Rugo HS, Campone M, Amadori D, Wardley AM, Aldrighetti D, Conte PF, Liu D, Mudenda B, McHenry MB, Pivot XB. Randomized phase II study of weekly versus every 3 week ixabepilone plus bevacizumab (ixa/bev) versus paclitaxel plus bev(pac/bev) as first-line therapy for metastatic breast cancer (MBC): final results. J Clin Oncol. 2010;28(Suppl 15):1040.
Kossoff EB, Ngamphaiboon N, Laudico TJ, O’Connor TL. Weekly ixabepilone administration in heavily pretreated metastatic breast cancer patients. Med Oncol. 2011;28(Suppl 1):S115–20.
Blum JL, Jones SE, Buzdar AU, et al. Multicenter phase II study of capecitabine in paclitaxel refractory metastatic breast cancer. J Clin Oncol. 1999;17:485–93.
Blum JL, Dees C, Chacko A, et al. Phase II trial of capecitabine and weekly paclitaxel as first-line therapy for metastatic breast cancer. J Clin Oncol. 2006;24:4384–90.
Talbot DC, Moiseyenko V, Van Belle S, et al. Randomised, phase II trial comparing oral capecitabine (Xeloda®) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer. 2002;86:1367–72.
Kuznetsov G, Towle MJ, Cheng H, Kawamura T, TenDyke K, Liu D, Kishi Y, Yu MJ, Littlefield BA. Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res. 2004;64:5760–6.
Towle MJ, Salvato KA, Budrow J, Wels BF, Kuznetsov G, Aalfs KK, Welsh S, Zheng W, Seletsky BM, Palme MH, Habgood GJ, Singer LA, Dipietro LV, Wang Y, Chen JJ, Quincy DA, Davis A, Yoshimatsu K, Kishi Y, Yu MJ, Littlefield BA. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 2001;61:1013–21.
Acknowledgments
The authors thank Wenxia Sun for data supply, Jing Ren for statistical analysis, Kaishun Wang for unit conversion of outcomes and Li Guo for English translation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Neither the entire paper nor any part of its content has been published or has been accepted elsewhere.It is not being submitted to any other journal. All authors have seen the manuscript and approved to submit to your journal. All authors do not have any financial relationship with a biotechnology manufacturer, a pharmaceutical company, or other commercial entity that has an interest in the subject matter or materials discussed in the manuscript.
About this article
Cite this article
Li, J., Ren, J. & Sun, W. Systematic review of ixabepilone for treating metastatic breast cancer. Breast Cancer 24, 171–179 (2017). https://doi.org/10.1007/s12282-016-0717-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-016-0717-0