Abstract
Background
In many trials (including E2100), bevacizumab (Bmab) monotherapy has been continued if toxicity of paclitaxel (PTX) becomes unacceptable during combined treatment with Bmab and PTX. When progression occurs on Bmab monotherapy, one possible option is re-induction with the combination of Bmab and PTX (rBP therapy), because PTX was previously stopped due to toxicity rather than progression. However, we have no data about rBP therapy. Therefore, we investigated the efficacy and safety of rBP therapy in this study.
Methods
We retrospectively investigated 46 patients who started Bmab and PTX between October 2011 and April 2013 at our institution.
Results
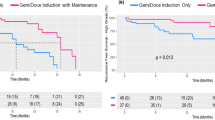
After induction with Bmab and PTX, 19 patients subsequently received Bmab monotherapy and 12 patients received rBP therapy. The overall response rate and clinical benefit rate of rBP therapy was 25 % (3/12) and 58 % (7/12), respectively, while the median time to failure of rBP therapy was 174 days (95 % CI 49–273). The median overall survival time of the 46 patients was 777 days (95 % CI 543–NA). Adverse events of grade 3 or worse associated with rBP therapy were neutropenia (25 %), fatigue (8 %), and gastrointestinal bleeding (8 %).
Conclusions
This is the first report about rBP therapy, which was found to be both safe and effective. The OS of all 46 patients in this study (including 12 patients given rBP therapy) was better than in past reports. Selecting rBP therapy for patients with progression on Bmab monotherapy might have contributed to better overall survival, but a randomized controlled trial will be needed to confirm these findings.




Similar content being viewed by others
References
Hurwitz H, Fehrenbacher L, Novtony W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–42.
Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel carboplatin alone or with bevacizumab for non small-cell lung cancer. N Engl J Med. 2006;355:2542–50.
Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–76.
Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–96.
Hershman DL, Lacchetti C, Dworkin RH, Smith EML, Bleeker J, Cavaletti G, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology and Clinical Practice. J Clin Oncol. 2014;32:1941–67.
Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novonty WF, et al. A Phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30:117–24.
Miles DW, Chan A, Dirix LY, Cortés J, Pivot X, Tomczak P, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2–negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–47.
von Minckwitz G, Puglisi F, Cortes J, Vrdoljak E, Marschner N, Zielinski C, et al. Bevacizumab plus chemotherapy versus chemotherapy alone as second-line treatment for patients with HER2-negative locally recurrent or metastatic breast cancer after first-line treatment with bevacizumab plus chemotherapy (TANIA): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:1269–78.
Brufsky AM, Hurvitz S, Perez E, Swamy R, Valero V, O’Neill V, et al. RIBBON-2: a randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2–negative metastatic breast cancer. J Clin Oncol. 2011;29:4286–93.
Yoshinami T, et al. SABCS 2013 Abst#OT3-1-01.
Saji S, et al. ASCO 2014 Abst#TPS657.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Tetsuhiro Yoshinami has received honoraria for lecture from Chugai. Takahiro Nakayama has received honoraria for lecture from Chugai, Novartis and Astra Zeneca, and research funding from Chugai.
About this article
Cite this article
Yoshinami, T., Yagi, T., Okuno, J. et al. Efficacy and safety of re-induction therapy with bevacizumab and paclitaxel for metastatic breast cancer. Breast Cancer 24, 147–151 (2017). https://doi.org/10.1007/s12282-016-0686-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-016-0686-3