Abstract
Background
Although carcinoembryonic antigen (CEA) and cancer antigen 15-3 (CA15-3) are useful tumor markers (TMs) in metastatic breast cancer (MBC), circulating tumor cells (CTCs) are also detected in patients with advanced or metastatic breast cancer. We analyzed CTCs in MBC patients in order to establish the optimal cut-off value, to evaluate the prognostic utility of CTC count, and to clarify whether CTC count could provide information in addition to CEA and CA15-3.
Methods
We studied 98 MBC patients enrolled between June 2007 and March 2013. To quantify CTCs, 7.5 ml of blood was collected and CEA and CA15-3 were measured simultaneously. CTCs were counted using the CellSearch™ System. The CTC count was dichotomized as 0 (CTC-negative) or ≥1 (CTC-positive). The clinical significance of CTCs was evaluated in terms of its relationship with levels of CEA and CA15-3. Associations between qualitative variables were evaluated using the chi-square test. In order to evaluate the predictive value of CTCs for advanced or metastatic breast cancer, multivariate Cox proportional hazards modeling was used to calculate hazard ratios.
Results
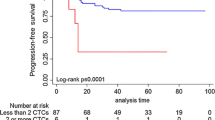
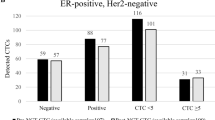
With a CTC cut-off value of 1, there were 53 (54.1 %) CTC-negative patients and 45 (45.9 %) CTC-positive patients. Patients in the CTC-positive group had worse survival than those in the CTC-negative group (p < 0.0001). Seventy-one patients (72.4 %) had TM data at the time of CTC testing. To study the relationship between CTCs and TMs, we divided patients into normal TM and high TM groups. In the normal TM group, the CTC-negative patients had statistically significant survival than the CTC-positive patients (p = 0.005). The data suggested that CTC count could provide additional prognostic information beyond TMs for advanced/metastatic breast cancer. In multivariate analysis, the only significant predictor of overall survival was CTC ≥ 1 (hazard ratio, 3.026; 95 % confidence interval 1.350–6.784).
Conclusion
We found that a CTC cut-off value of 1 is appropriate in patients with advanced/metastatic breast cancer. CTCs could yield additional information beyond CEA and CA15-3.




Similar content being viewed by others
References
Miller MC, Doyle GV, Terstappen LW. Significance of circulating tumor cells detected by the cell search system in patients with metastatic breast colorectal and prostate cancer. J Oncol. 2010;2010:617421.
Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21:729–33.
Fehm T, Sauerbrei W. Information from CTC measurements for metastatic breast cancer prognosis-we should do more than selecting an “optimal cut point”. Breast Cancer Res Treat. 2010;122:219–20.
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12:4218–24.
Coughlin SS, Ekwueme DU. Breast cancer as a global health concern. Cancer Epidemiol. 2009;33:315–8.
Stathopoulou A, Vlachonikolis I, Mavroudis D, Perraki M, Kouroussis C, Apostolaki S, et al. Molecular detection of cytokeratin-19-positive cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic significance. J Clin Oncol. 2002;20:3404–12.
Xenidis N, Perraki M, Kafousi M, Apostolaki S, Bolonaki I, Stathopoulou A, et al. Predictive and prognostic value of peripheral blood cytokeratin-19 mRNA-positive cells detected by real-time polymerase chain reaction in node-negative breast cancer patients. J Clin Oncol. 2006;24:3756–62.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91.
Martin M, Garcia-Saenz JA, Maestro De las Casas ML, Vidaurreta M, Puente J, Veganzones S, et al. Circulating tumor cells in metastatic breast cancer: timing of blood extraction for analysis. Anticancer Res. 2009;29:4185–7.
Ramirez JM, Fehm T, Orsini M, Cayrefourcq L, Maudelonde T, Pantel K, et al. Prognostic relevance of viable circulating tumor cells detected by EPISPOT in metastatic breast cancer patients. Clin Chem. 2014;60:214–21.
Kaiser J. Medicine. Cancer’s circulation problem. Science. 2010;327:1072–4.
Kallergi G, Konstantinidis G, Markomanolaki H, Papadaki MA, Mavroudis D, Stournaras C, et al. Apoptotic circulating tumor cells in early and metastatic breast cancer patients. Mol Cancer Ther. 2013;12:1886–95.
Pierga JY, Hajage D, Bachelot T, Delaloge S, Brain E, Campone M, et al. High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Ann Oncol. 2012;23:618–24.
Giordano A, Egleston BL, Hajage D, Bland J, Hortobagyi GN, Reuben JM, et al. Establishment and validation of circulating tumor cell-based prognostic nomograms in first-line metastatic breast cancer patients. Clin Cancer Res. 2013;19:1596–602.
Babayan A, Hannemann J, Spotter J, Muller V, Pantel K, Joosse SA. Heterogeneity of estrogen receptor expression in circulating tumor cells from metastatic breast cancer patients. PLoS ONE. 2013;8:e75038.
Roop RP, Naughton MJ, Van Poznak C, Schneider JG, Lammers PE, Pluard TJ, et al. A randomized phase II trial investigating the effect of platelet function inhibition on circulating tumor cells in patients with metastatic breast cancer. Clin Breast Cancer. 2013;13:409–15.
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging—predicting overall survival in metastatic breast cancer. Clin Cancer Res. 2006;12:6403–9.
Friedlander TW, Premasekharan G, Paris PL. Looking back, to the future of circulating tumor cells. Pharmacol Ther. 2013. doi:10.1016/j.pharmthera.2013.12.011.
Chimonidou M, Kallergi G, Georgoulias V, Welch DR, Lianidou ES. Breast cancer metastasis suppressor-1 promoter methylation in primary breast tumors and corresponding circulating tumor cells. Mol Cancer Res. 2013;11:1248–57.
Botteri E, Sandri MT, Bagnardi V, Munzone E, Zorzino L, Rotmensz N, et al. Modeling the relationship between circulating tumour cells number and prognosis of metastatic breast cancer. Breast Cancer Res Treat. 2010;122:211–7.
Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol. 2009;27:5153–9.
Nakamura S, Yagata H, Ohno S, Yamaguchi H, Iwata H, Tsunoda N, et al. Multi-center study evaluating circulating tumor cells as a surrogate for response to treatment and overall survival in metastatic breast cancer. Breast Cancer. 2010;17:199–204.
Braun S, Hepp F, Kentenich CR, Janni W, Pantel K, Riethmuller G, et al. Monoclonal antibody therapy with edrecolomab in breast cancer patients: monitoring of elimination of disseminated cytokeratin-positive tumor cells in bone marrow. Clin Cancer Res. 1999;5:3999–4004.
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (21591680) in Japan.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Shiomi-Mouri, Y., Kousaka, J., Ando, T. et al. Clinical significance of circulating tumor cells (CTCs) with respect to optimal cut-off value and tumor markers in advanced/metastatic breast cancer. Breast Cancer 23, 120–127 (2016). https://doi.org/10.1007/s12282-014-0539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-014-0539-x