Abstract
Purpose
In the past decade, JBCRG has conducted three studies of neoadjuvant chemotherapy which have examined sequential combination of fluorouracil, epirubicin and cyclophosphamide, and docetaxel. The present study is a pooled analysis of these studies performed to determine the prognostic significance of pathologic complete response (pCR) and predictive variables for pCR.
Methods
A total of 353 patients were included. pCR was defined as the absence of invasive cancer or only a few remaining isolated cancer cells in the breast (quasi-pCR, QpCR).
Results
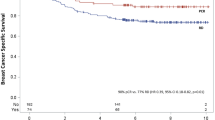
Disease-free survival (DFS) and overall survival (OS) were not significantly different among studies, and patients who achieved a QpCR had significantly better prognosis (DFS, p < 0.001; OS, p = 0.002). Patients with triple-negative (TN) tumors had worse prognosis than patients with the other subtypes (DFS, p = 0.03; OS, p = 0.10). A Cox proportional hazards model showed node-positive, TN, and QpCR were the significant predictors for DFS and OS among study, age, tumor size, nuclear grade, nodal status, subtype, clinical response, and pathologic response (DFS; node-positive, HR = 2.29, p = 0.001; TN, HR = 3.39, p < 0.001; QpCR, HR = 0.27, p < 0.001: OS; node-positive, HR = 3.05, p = 0.003; TN, HR = 4.92, p < 0.001; QpCR, HR = 0.12, p < 0.001). In a logistic regression analysis, subtype and clinical response before surgery were the significant predictive variables for QpCR (luminal/Her2-positive, odds ratio (OR) = 4.15, p = 0.002; Her2-positive, OR = 6.24, p < 0.001; TN, OR = 4.24, p < 0.001; clinical response before surgery, OR = 2.41, p = 0.019).
Conclusions
This study confirmed the prognostic significance of QpCR and nodal status and the predictive and prognostic significance of subtype in neoadjuvant chemotherapy.





Similar content being viewed by others
References
Kaufmann M, Hortobagyi GN, Goldhirsch A, Scholl S, Makris A, Valagussa P, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: an update. J Clin Oncol. 2006;24:1940–9.
Kaufmann M, von Minckwitz G, Bear HD, Buzdar A, McGale P, Bonnefoi H, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006. Ann Oncol. 2007;18:1927–34.
von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–804.
Cortazar P, Zhang L, Untch M, Mehta K, Costantino J, Wolmark N, et al. Meta-analysis results from the collaborative trials in neoadjuvant breast cancer (CTNeoBC) S1–11. Cancer Res. 2012;72.
von Minckwitz G, Untch M, Nuesch E, Loibl S, Kaufmann M, Kummel S, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011;125:145–56.
Kuroi K, Toi M, Tsuda H, Kurosumi M, Akiyama F. Issues in the assessment of the pathologic effect of primary systemic therapy for breast cancer. Breast Cancer. 2006;13:38–48.
Toi M, Nakamura S, Kuroi K, Iwata H, Ohno S, Masuda N, et al. Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat. 2008;110:531–9.
Nakamura S, Masuda S, Iwata H, Toi M, Kuroi K, Kurozumi M, et al. Phase II trial of fluorouracil, epirubicin, cyclophosphamide (FEC) followed by docetaxel 100 mg/m2 in primary operable breast cancer-JBCRG02-. Jpn J Breast Cancer. 2008;23:111–7.
Iwata H, Sato N, Masuda N, Nakamura S, Yamamoto N, Kuroi K, et al. Docetaxel followed by fluorouracil/epirubicin/cyclophosphamide as neoadjuvant chemotherapy for patients with primary breast cancer. Jpn J Clin Oncol. 2011;41:867–75.
Kurosumi M, Akashi-Tanaka S, Akiyama F, Komoike Y, Mukai H, Nakamura S, et al. Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version). Breast Cancer. 2008;15:5–7.
Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg. 1995;180:297–306.
Sinn HP, Schmid H, Junkermann H, Huober J, Leppien G, Kaufmann M, et al. Histologic regression of breast cancer after primary (neoadjuvant) chemotherapy. Geburtshilfe Frauenheilkd. 1994;54:552–8.
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25:4414–22.
Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–27.
Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342–54.
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–84.
Liedtke C, Mazouni C, Hess KR, Andre F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26:1275–81.
Ohno S, Kuroi K, Toi M. An overview of the Japan Breast Cancer Research Group (JBCRG) activities. Breast Cancer. 2013 Mar 15. (Epub ahead of print).
Kuroi K, Kashiwa K, Toi M, Nakamura S, Iwata H, Ohno S, et al. Japan Breast Cancer Research Group (JBCRG). Clin Oncol. 2010;6:360–8.
Manton DJ, Chaturvedi A, Hubbard A, Lind MJ, Lowry M, Maraveyas A, et al. Neoadjuvant chemotherapy in breast cancer: early response prediction with quantitative MR imaging and spectroscopy. Br J Cancer. 2006;94:427–35.
Rousseau C, Devillers A, Sagan C, Ferrer L, Bridji B, Campion L, et al. Monitoring of early response to neoadjuvant chemotherapy in stage II and III breast cancer by [18F]fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2006;24:5366–72.
Acknowledgments
The JBCRG-01 study was supported by the Osaka Cancer Research Foundation and the Advanced Clinical Research Organization; JBCRG-02 and JBCRG-03 studies were supported by the Advanced Clinical Research Organization. The authors gratefully thank the patients who participated in the JBCRG-01, JBCRG-02, and JBCRG-03 studies. The authors also thank Mrs Kiyomi Kashiwa and Mrs Aya Maruyama for their support and data management.
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kuroi, K., Toi, M., Ohno, S. et al. Prognostic significance of subtype and pathologic response in operable breast cancer; a pooled analysis of prospective neoadjuvant studies of JBCRG. Breast Cancer 22, 486–495 (2015). https://doi.org/10.1007/s12282-013-0511-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-013-0511-1