Abstract
Background
The effectiveness of neoadjuvant chemotherapy is evaluated on the basis of pathological responses and survival outcome, because achievement of a pathological complete response (pCR) is a good predictor of long-term survival. However, few studies have assessed the survival of breast cancer patients who received neoadjuvant chemotherapy including trastuzumab.
Methods
The records of 161 breast cancer patients who received neoadjuvant chemotherapy between January 2006 and December 2011 were retrospectively reviewed. The patients were categorized into 4 subgroups on the basis of the status of the estrogen receptor (ER), the progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). HER2-positive patients received trastuzumab-based regimens. Pathological responses and survival were analyzed on the basis of breast cancer subtypes.
Results
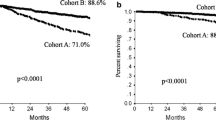
The pCR results obtained were: luminal A and B (ER and/or PR-positive, HER2-negative), 6.3 % (5/79 cases); luminal–HER2 hybrid (ER and/or PR-positive, HER2-positive), 25.0 % (5/20 cases); HER2-enriched (ER and PR-negative, HER2-positive), 63.0 % (17/27 cases); and triple-negative (ER and PR-negative, HER2-negative), 25.7 % (9/35 cases). Achievement of pCR was a good predictor of disease-free survival in the HER2-enriched group. Overall survival of patients with pCR was slightly, but not significantly, better in the HER2-enriched and triple-negative subgroups.
Conclusion
Responses and survival after neoadjuvant chemotherapy including trastuzumab of patients with HER2-positive tumors differed among disease subtypes. Our findings suggest that disease subtype is an important determinant of the efficacy of neoadjuvant chemotherapy.





Similar content being viewed by others
References
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85. doi:10.1200/JCO.2007.15.0235.
van Nes JG, Putter H, Julien JP, Tubiana-Hulin M, van de Vijver M, Bogaerts J, et al. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up; clinical and translational results from the EORTC trial 10902. Breast Cancer Res Treat. 2009;115(1):101–13. doi:10.1007/s10549-008-0050-1.
Kong X, Moran MS, Zhang N, Haffty B, Yang Q. Meta-analysis confirms achieving pathological complete response after neoadjuvant chemotherapy predicts favourable prognosis for breast cancer patients. Eur J Cancer. 2011;47(14):2084–90. doi:10.1016/j.ejca.2011.06.014.
Gianni L, Baselga J, Eiermann W, Guillem Porta V, Semiglazov V, Lluch A, et al. Feasibility and tolerability of sequential doxorubicin/paclitaxel followed by cyclophosphamide, methotrexate, and fluorouracil and its effects on tumor response as preoperative therapy. Clin Cancer Res. 2005;11(24 Pt 1):8715–21. doi:10.1158/1078-0432.CCR-05-0539.
Valachis A, Mauri D, Polyzos NP, Chlouverakis G, Mavroudis D, Georgoulias V. Trastuzumab combined to neoadjuvant chemotherapy in patients with HER2-positive breast cancer: a systematic review and meta-analysis. Breast. 2011;20(6):485–90. doi:10.1016/j.breast.2011.06.009.
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–84. doi:10.1016/S0140-6736(09)61964-4.
Rodenhuis S, Mandjes IA, Wesseling J, van de Vijver MJ, Peeters MJ, Sonke GS, et al. A simple system for grading the response of breast cancer to neoadjuvant chemotherapy. Ann Oncol. 2010;21(3):481–7. doi:10.1093/annonc/mdp348.
Yin W, Jiang Y, Shen Z, Shao Z, Lu J. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PLoS ONE. 2011;6(6):e21030. doi:10.1371/journal.pone.0021030.
Harris CA, Ward RL, Dobbins TA, Drew AK, Pearson S. The efficacy of HER2-targeted agents in metastatic breast cancer: a meta-analysis. Ann Oncol. 2011;22(6):1308–17. doi:10.1093/annonc/mdq593.
Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–47. doi:10.1093/annonc/mdr304.
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–85. doi:10.1200/JCO.2005.07.032.
Untch M, Fasching PA, Konecny GE, Hasmüller S, Lebeau A, Kreienberg R, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–7. doi:10.1200/JCO.2010.31.4930.
Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024–31. doi:10.1200/JCO.2009.23.8451.
Buzdar AU, Valero V, Theriault R, Frye D, Green M, Booser D, et al. Pathological complete response to chemotherapy is related to hormone receptor status. Breast Cancer Res Treat. 2003;82:S69.
Bhargava R, Beriwal S, Dabbs DJ, Ozbek U, Soran A, Johnson RR, et al. Immunohistochemical surrogate markers of breast cancer molecular classes predicts response to neoadjuvant chemotherapy: a single institutional experience with 359 cases. Cancer. 2010;116(6):1431–9. doi:10.1002/cncr.24876.
Toi M, Nakamura S, Kuroi K, Iwata H, Ohno S, Masuda N, et al. Phase II study of preoperative sequential FEC and docetaxel predicts of pathological response and disease free survival. Breast Cancer Res Treat. 2008;110(3):531–9. doi:10.1007/s10549-007-9744-z.
Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, et al. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369(9555):29–36. doi:10.1016/S0140-6736(07)60028-2.
Brufsky A, Lembersky B, Schiffman K, Lieberman G, Paton VE. Hormone receptor status does not affect the clinical benefit of trastuzumab therapy for patients with metastatic breast cancer. Clin Breast Cancer. 2005;6(3):247–52. doi:10.3816/CBC.2005.n.027.
Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. doi:10.1056/NEJMoa052306.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ohzawa, H., Sakatani, T., Niki, T. et al. Pathological responses and survival of patients with human epidermal growth factor receptor 2-positive breast cancer who received neoadjuvant chemotherapy including trastuzumab. Breast Cancer 21, 563–570 (2014). https://doi.org/10.1007/s12282-012-0424-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-012-0424-4