Abstract
Background
The purpose of this study was to compare the accuracy of volumetric (3D) measurements with that of unidimensional (1D) measurements by response evaluation criteria in solid tumors 1.1 (RECIST 1.1) in patients with breast cancer before and after neoadjuvant chemotherapy.
Methods
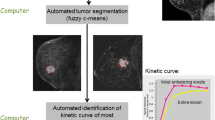
The study included 48 patients with breast cancer who underwent neoadjuvant chemotherapy. Dynamic contrast-enhanced magnetic resonance imaging was performed before the first cycle of chemotherapy and after the completion of the planned chemotherapy. The longest diameter and volume of each target lesion were measured using a TeraRecon Aquarius workstation (San Mateo, CA). Response was assessed both by using the RECIST 1.1 and volumetric criteria. Histologic response was assessed using the Sataloff criteria. The agreements between the two measures and the histologic response were analyzed statistically.
Results
In monitoring the response to neoadjuvant chemotherapy, the 1D and 3D measurements showed “good agreement” (κ = 0.610) for the treatment response categories and “moderate agreement” (κ = 0.565) for the responder/non-responder categories. Disagreement was observed in 9 out of 48 comparisons (18.75 %). The percent agreement of the 1D measurement of residual lesions (79.17 %) with the pathology was higher than that by volumetric measurement (70.83 %), but there was no statistically significant difference (p = 0.35). Both the 1D (rho = 0.67, p < 0.0001) and 3D measurements (rho = 0.52, p < 0.0001) showed a moderate degree of linear correlation with the pathologic diameter of residual lesions.
Conclusion
There was generally good agreement between the 1D and 3D measurements and moderate predictive value using either approach for predicting pathological response.




Similar content being viewed by others
References
Kaufmann M, von Minckwitz G, Smith R, Valero V, Gianni L, Eiermann W, et al. International expert panel on the use of primary (preoperative) systemic treatment of operable breast cancer: review and recommendations. J Clin Oncol. 2003;21:2600–8.
Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–94.
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–93.
Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–85.
Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102.
Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21:4165–74.
Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–85.
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–22.
Jeruss JS, Mittendorf EA, Tucker SL, Gonzalez-Angulo AM, Buchholz TA, Sahin AA, et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J Clin Oncol. 2008;26(2):246–52.
Padhani AR, Hayes C, Assersohn L, Powles T, Makris A, Suckling J, et al. Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical results. Radiology. 2006;239:361–74.
Balu-Maestro C, Chapellier C, Bleuse A, Chanalet I, Chauvel C, Largillier R. Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat. 2002;72(2):145–52.
Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kühn T. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol. 2002;12(7):1711–9.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42:1031–9.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Bogaerts J, Ford R, Sargent D, Schwartz LH, Rubinstein L, Lacombe D, et al. Individual patient data analysis to assess modifications to the RECIST criteria. Eur J Cancer. 2009;45:248–60.
Moskowitz CS, Jia X, Schwartz LH, Gönen M. A simulation study to evaluate the impact of the number of lesions measured on response assessment. Eur J Cancer. 2009;45:300–10.
Schwartz LH, Bogaerts J, Ford R, Shankar L, Therasse P, Gwyther S, et al. Evaluation of lymph nodes with RECIST 1.1. Eur J Cancer. 2009;45:261–7.
Suzuki C, Jacobsson H, Hatschek T, Torkzad MR, Bodén K, Eriksson-Alm Y, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008;28:329–44.
Sataloff DM, Mason BA, Prestipino AJ, Seinige UL, Lieber CP, Baloch Z. Pathologic response to induction chemotherapy in locally advanced carcinoma of the breast: a determinant of outcome. J Am Coll Surg. 1995;180(3):297–306.
Tran LN, Brown MS, Goldin JG, Yan X, Pais RC, McNitt-Gray MF, et al. Comparison of treatment response classifications between unidimensional, bidimensional, and volumetric measurements of metastatic lung lesions on chest computed tomography. Acad Radiol. 2004;11:1355–60.
Warren KE, Patronas N, Aikin AA, Albert PS, Balis FM. Comparison of one-, two- and three-dimensional measurements of childhood brain tumours. J Nat Cancer Inst. 2001;93:1401–5.
Sze G, Mehta MP, Schutlz CJ, Ford JM, Roa WH, Leibenhaut M, et al. Radiologic response evaluation of brain metastases: unidimensional World Health Organization (WHO) response evaluation criteria in solid tumours (RECIST) vs bidimensional or 3-dimensional criteria. Proc Am Soc Clin Oncol 2001; 20:59 [abstr 234].
Galanis E, Maurer MJ, Ballman KV, O’Fallon JR, Sykora R, Castillo R, et al. Validation of neuroradiologic response assessment in gliomas: RECIST (1D) versus 2D measurements versus computer-assisted tumour area versus volume. Proc Am Soc Clin Oncol 2003; 22:106 [abstr 423].
Shah G, Kesari S, Xu R, Henson J, Batchelor T, Hochberg F, Oneill A, et al. Comparison of 1D, 2D, 3D and volumetric parameters in measuring tumour response in high-grade gliomas in adults. Proc Am Soc Clin Oncol 2004; 23:112 [abstr 1523].
Sohaib SA, Turner B, Hanson JA, Farquharson M, Oliver RT, Reznek RH. CT assessment of tumour response to treatment: comparison of linear, cross-sectional and volumetric measures of tumour size. Br J Radiol. 2000;73:1178–84.
Sebastian S, Fabio F, Sverzellati N, Chiari G, Colomer R. 3D assessment of Lymph nodes versus RECIST 1.1. Acad Radiol. 2011;18(3):391–4.
Galanis E, Buckner JC, Maurer MJ, Sykora R, Castillo R, Ballman KV, et al. Validation of neuroradiologic response assessment in gliomas: measurement by RECIST, two-dimensional, computer-assisted tumor area, and computer-assisted tumor volume methods. Neuro Oncol. 2006;8(2):156–65.
Shah GD, Kesari S, Xu R, Batchelor TT, O’Neill AM, Hochberg FH, et al. Comparison of linear and volumetric criteria in assessing tumor response in adult high-grade gliomas. Neuro Oncol. 2006;8(1):38–46.
Marten K, Auer F, Schmidt S, Kohl G, Rummeny EJ, Engelke C. Inadequacy of manual measurements compared to automated CT volumetry in assessment of treatment response of pulmonary metastases using RECIST criteria. Eur Radiol. 2006;16(4):781–90.
Lyou CY, Cho N, Kim SM, Jang M, Park JS, Baek SY, et al. Computer-aided evaluation of breast MRI for the residual tumor extent and response monitoring in breast cancer patients receiving neoadjuvant chemotherapy. Korean J Radiol. 2011;12(1):34–43.
Alderliesten T, Schlief A, Peterse J, Loo C, Teertstra H, Muller S, et al. Validation of semiautomatic measurement of the extent of breast tumors using contrast-enhanced magnetic resonance imaging. Invest Radiol. 2007;42(1):42–9.
Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Tripathy D, Wolverton DS, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. Am J Roentgenol. 2005;184:1774–81.
Martincich L, Montemurro F, De Rosa G, Marra V, Ponzone R, Cirillo S, et al. Monitoring response to primary chemotherapy in breast cancer using dynamic contrast-enhanced magnetic resonance imaging. Breast Cancer Res Treat. 2004;83(1):67–76.
Lorenzon M, Zuiani C, Londero V, Linda A, Furlan A, Bazzocchi M. Assessment of breast cancer response to neoadjuvant chemotherapy: is volumetric MRI a reliable tool? Eur J Radiol. 2009;71(1):82–8.
Rieber A, Zeitler H, Rosenthal H, Görich J, Kreienberg R, Brambs HJ, et al. MRI of breast cancer: influence of chemotherapy on sensitivity. Br J Radiol. 1997;70:452–8.
Wasser K, Sinn HP, Fink C, Klein SK, Junkermann H, Lüdemann HP, et al. Accuracy of tumor size measurement in breast cancer using MRI is influenced by histological regression induced by neoadjuvant chemotherapy. Eur Radiol. 2003;13:1213–23.
Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. Am J Roentgenol. 2002;179(5):1193–9.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
An, Y.Y., Kim, S.H., Kang, B.J. et al. MRI volume measurements compared with the RECIST 1.1 for evaluating the response to neoadjuvant chemotherapy for mass-type lesions. Breast Cancer 21, 316–324 (2014). https://doi.org/10.1007/s12282-012-0388-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12282-012-0388-4