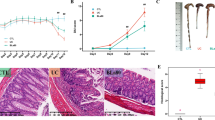
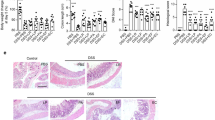
Abstract
Ulcerative colitis, a major form of inflammatory bowel disease (IBD) associated with chronic colonic inflammation, may be induced via overreactive innate and adaptive immune responses. Restoration of gut microbiota abundance and diversity is important to control the pathogenesis. Lactobacillus spp., well-known probiotics, ameliorate IBD symptoms via various mechanisms, including modulation of cytokine production, restoration of gut tight junction activity and normal mucosal thickness, and alterations in the gut microbiota. Here, we studied the effects of oral administration of Lactobacillus rhamnosus (L. rhamnosus) KBL2290 from the feces of a healthy Korean individual to mice with DSS-induced colitis. Compared to the dextran sulfate sodium (DSS) + phosphate-buffered saline control group, the DSS + L. rhamnosus KBL2290 group evidenced significant improvements in colitis symptoms, including restoration of body weight and colon length, and decreases in the disease activity and histological scores, particularly reduced levels of pro-inflammatory cytokines and an elevated level of anti-inflammatory interleukin-10. Lactobacillus rhamnosus KBL2290 modulated the levels of mRNAs encoding chemokines and markers of inflammation; increased regulatory T cell numbers; and restored tight junction activity in the mouse colon. The relative abundances of genera Akkermansia, Lactococcus, Bilophila, and Prevotella increased significantly, as did the levels of butyrate and propionate (the major short-chain fatty acids). Therefore, oral L. rhamnosus KBL2290 may be a useful novel probiotic.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Alatab, S., Sepanlou, S. G., Ikuta, K., Vahedi, H., Bisignano, C., Safiri, S., Sadeghi, A., Nixon, M. R., Abdoli, A., Abolhassani, H., et al. (2020). The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. The Lancet Gastroenterology and Hepatology, 5, 17–30.
Almagro-Moreno, S., & Boyd, E. F. (2009). Sialic acid catabolism confers a competitive advantage to pathogenic Vibrio cholerae in the mouse intestine. Infection and Immunity, 77, 3807–3816.
Belkaid, Y., & Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cell, 157, 121–141.
Berry, D., Schwab, C., Milinovich, G., Reichert, J., Mahfoudh, K. B., Decker, T., Engel, M., Hai, B., Hainzl, E., Heider, S., et al. (2012). Phylotype-level 16S rRNA analysis reveals new bacterial indicators of health state in acute murine colitis. ISME Journal, 6, 2091–2106.
Blander, J. M., Longman, R. S., Iliev, I. D., Sonnenberg, G. F., & Artis, D. (2017). Regulation of inflammation by microbiota interactions with the host. Nature Immunology, 18, 851–860.
Chassaing, B., Aitken, J. D., Malleshappa, M., & Vijay-Kumar, M. (2014). Dextran sulfate sodium (DSS)-induced colitis in mice. Current Protocols in Immunology, 104, 15.25.1-15.25.14.
Chelakkot, C., Ghim, J., & Ryu, S. H. (2018). Mechanisms regulating intestinal barrier integrity and its pathological implications. Experimental & Molecular Medicine, 50, 1–9.
Collado, M. C., Derrien, M., Isolauri, E., de Vos, W. M., & Salminen, S. (2007). Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Applied and Environment Microbiology, 73, 7767–7770.
Derrien, M., Vaughan, E. E., Plugge, C. M., & de Vos, W. M. (2004). Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. International Journal of Systematic and Evolutionary Microbiology, 54, 1469–1476.
Duranti, S., Gaiani, F., Mancabelli, L., Milani, C., Grandi, A., Bolchi, A., Santoni, A., Lugli, G. A., Ferrario, C., Mangifesta, M., et al. (2016). Elucidating the gut microbiome of ulcerative colitis: Bifidobacteria as novel microbial biomarkers. FEMS Microbiology Ecology, 92, 191.
Duvallet, C., Gibbons, S. M., Gurry, T., Irizarry, R. A., & Alm, E. J. (2017). Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nature Communications, 8, 1784.
Frank, D. N., Amand, A. L. S., Feldman, R. A., Boedeker, E. C., Harpaz, N., & Pace, N. R. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America, 104, 13780–13785.
Fukuda, M., Kanauchi, O., Araki, Y., Andoh, A., Mitsuyama, K., Takagi, K., Toyonaga, A., Sata, M., Fujiyama, Y., Fukuoka, M., et al. (2002). Prebiotic treatment of experimental colitis with germinated barley foodstuff: A comparison with probiotic or antibiotic treatment. International Journal of Molecular Medicine, 9, 65–70.
Gevers, D., Kugathasan, S., Denson, L. A., Vázquez-Baeza, Y., Van Treuren, W., Ren, B., Schwager, E., Knights, D., Song, S. J., Yassour, M., et al. (2014). The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host & Microbe, 15, 382–392.
Ghaleb, A. M., McConnell, B. B., Kaestner, K. H., & Yang, V. W. (2011). Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Krüppel-like factor 4 gene. Developmental Biology, 349, 310–320.
Halfvarson, J., Brislawn, C. J., Lamendella, R., Vázquez-Baeza, Y., Walters, W. A., Bramer, L. M., D’amato, M., Bonfiglio, F., McDonald, D., Gonzalez, A., et al. (2017). Dynamics of the human gut microbiome in inflammatory bowel disease. Nature Microbiology, 2, 17004.
Han, D. H., Kim, W. K., Lee, C., Park, S., Lee, K., Jang, S. J., & Ko, G. (2022). Co-administration of Lactobacillus gasseri KBL697 and tumor necrosis factor-alpha inhibitor infliximab improves colitis in mice. Scientific Reports, 12, 9640.
Hooper, L. V., & Macpherson, A. J. (2010). Immune adaptations that maintain homeostasis with the intestinal microbiota. Nature Reviews Immunology, 10, 159–169.
Ito, R., Kita, M., Shin-Ya, M., Kishida, T., Urano, A., Takada, R., Sakagami, J., Imanishi, J., Iwakura, Y., Okanoue, T., et al. (2008). Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochemical and Biophysical Research Communications, 377, 12–16.
Jang, Y. J., Kim, W. K., Han, D. H., Lee, K., & Ko, G. (2019). Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes, 10, 696–711.
Kedia, S., Ghosh, T. S., Jain, S., Desigamani, A., Kumar, A., Gupta, V., Bopanna, S., Yadav, D. P., Goyal, S., Makharia, G., et al. (2021). Gut microbiome diversity in acute severe colitis is distinct from mild to moderate ulcerative colitis. Journal of Gastroenterology and Hepatology, 36, 731–739.
Khan, I., Ullah, N., Zha, L., Bai, Y., Khan, A., Zhao, T., Che, T., & Zhang, C. (2019). Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens, 8, 126.
Khor, B., Gardet, A., & Xavier, R. J. (2011). Genetics and pathogenesis of inflammatory bowel disease. Nature, 474, 307–317.
Kim, W. K., Han, D. H., Jang, Y. J., Park, S., Jang, S. J., Lee, G., Han, H. S., & Ko, G. (2021). Alleviation of DSS-induced colitis via Lactobacillus acidophilus treatment in mice. Food & Function, 12, 340–350.
Kim, W. K., Jang, Y. J., Seo, B., Han, D. H., Park, S., & Ko, G. (2019). Administration of Lactobacillus paracasei strains improves immunomodulation and changes the composition of gut microbiota leading to improvement of colitis in mice. Journal of Functional Foods, 52, 565–575.
Kosiewicz, M. M., Zirnheld, A. L., & Alard, P. (2011). Gut microbiota, immunity, and disease: A complex relationship. Frontiers in Microbiology, 2, 180.
Li, C., Jiang, P., Wei, S., Xu, X., & Wang, J. (2020a). Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Molecular Cancer, 19, 116.
Li, Y., Yang, S., Lun, J., Gao, J., Gao, X., Gong, Z., Wan, Y., He, X., & Cao, H. (2020b). Inhibitory effects of the Lactobacillus rhamnosus GG effector protein HM0539 on inflammatory response through the TLR4/MyD88/NF-кB axis. Frontiers in Immunology, 11, 551449.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods, 25, 402–408.
Mangalam, A., Shahi, S. K., Luckey, D., Karau, M., Marietta, E., Luo, N., Choung, R. S., Ju, J., Sompallae, R., Gibson-Corley, K., et al. (2017). Human gut-derived commensal bacteria suppress CNS inflammatory and demyelinating disease. Cell Reports, 20, 1269–1277.
Mazmanian, S. K., Round, J. L., & Kasper, D. L. (2008). A microbial symbiosis factor prevents intestinal inflammatory disease. Nature, 453, 620–625.
Miyauchi, E., Morita, H., & Tanabe, S. (2009). Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. Journal of Dairy Science, 92, 2400–2408.
Morgan, X. C., Tickle, T. L., Sokol, H., Gevers, D., Devaney, K. L., Ward, D. V., Reyes, J. A., Shah, S. A., LeLeiko, N., Snapper, S. B., et al. (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biology, 13, R79.
Munyaka, P. M., Rabbi, M. F., Khafipour, E., & Ghia, J. E. (2016). Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. Journal of Basic Microbiology, 56, 986–998.
Neish, A. S. (2009). Microbes in gastrointestinal health and disease. Gastroenterology, 136, 65–80.
Nguyen, L. H., Örtqvist, A. K., Cao, Y., Simon, T. G., Roelstraete, B., Song, M., Joshi, A. D., Staller, K., Chan, A. T., Khalili, H., et al. (2020). Antibiotic use and the development of inflammatory bowel disease: A national case-control study in Sweden. The Lancet Gastroenterology and Hepatology, 5, 986–995.
Ott, S., Musfeldt, M., Wenderoth, D. F., Hampe, J., Brant, O., Fölsch, U. R., Timmis, K. N., & Schreiber, S. (2004). Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut, 53, 685–693.
Ottman, N., Reunanen, J., Meijerink, M., Pietilä, T. E., Kainulainen, V., Klievink, J., Huuskonen, L., Aalvink, S., Skurnik, M., et al. (2017). Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS ONE, 12, e0173004.
Ouwerkerk, J. P., Aalvink, S., Belzer, C., & de Vos, W. M. (2016). Akkermansia glycaniphila sp. nov., an anaerobic mucin-degrading bacterium isolated from reticulated python faeces. International Journal of Systematic and Evolutionary Microbiology, 66, 4614–4620.
Parada Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., Harmsen, H. J. M., Faber, K. N., & Hermoso, M. A. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology, 10, 277.
Paramsothy, S., Paramsothy, R., Rubin, D. T., Kamm, M. A., Kaakoush, N. O., Mitchell, H. M., & Castaño-Rodríguez, N. (2017). Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. Journal of Crohn's and Colitis, 11, 1180–1199.
Peñaloza, H. F., Noguera, L. P., Riedel, C. A., & Bueno, S. M. (2018). Expanding the current knowledge about the role of interleukin-10 to major concerning bacteria. Frontiers in Microbiology, 9, 2047.
Roselli, M., & Finamore, A. (2020). Use of synbiotics for ulcerative colitis treatment. Current Clinical Pharmacology, 15, 174–182.
Round, J. L., & Mazmanian, S. K. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology, 9, 313–323.
Rubtsov, Y. P., Rasmussen, J. P., Chi, E. Y., Fontenot, J., Castelli, L., Ye, X., Treuting, P., Siewe, L., Roers, A., Henderson, W. R., Jr., et al. (2008). Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity, 28, 546–558.
Sawant, K. V., Poluri, K. M., Dutta, A. K., Sepuru, K. M., Troshkina, A., Garofalo, R. P., & Rajarathnam, K. (2016). Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Scientific Reports, 6, 33123.
Schirmer, M., Franzosa, E. A., Lloyd-Price, J., McIver, L. J., Schwager, R., Poon, T. W., Ananthakrishnan, A. N., Andrews, E., Barron, G., Lake, K., et al. (2018). Dynamics of metatranscription in the inflammatory bowel disease gut microbiome. Nature Microbiology, 3, 337–346.
Seiffart, V., Zoeller, J., Klopfleisch, R., Wadwa, M., Hansen, W., Buer, J., Riedel, C., & Westendorf, A. M. (2015). IL10-deficiency in CD4+ T cells exacerbates the IFNγ and IL17 response during bacteria induced colitis. Cellular Physiology and Biochemistry, 36, 1259–1273.
Shahi, S. K., Freedman, S. N., Murra, A. C., Zarei, K., Sompallae, R., Gibson-Corley, K. N., Karandikar, N. J., Murray, J. A., & Mangalam, A. K. (2019). Prevotella histicola, a human gut commensal, is as potent as COPAXONE® in an animal model of multiple sclerosis. Frontiers in Immunology, 10, 462.
Shaw, K. A., Bertha, M., Hofmekler, T., Chopra, P., Vatanen, T., Srivatsa, A., Prince, J., Kumar, A., Sauer, C., Zwick, M. E., et al. (2016). Dysbiosis, inflammation, and response to treatment: A longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Medicine, 8, 75.
Shin, N. R., Lee, J. C., Lee, H. Y., Kim, M. S., Whon, T. W., Lee, M. S., & Bae, J. W. (2014). An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut, 63, 727–735.
Tan, J., McKenzie, C., Potamitis, M., Thorburn, A., Mackay, C., & Macia, L. (2014). The role of short-chain fatty acids in health and disease. Advances in Immunology, 121, 91–119.
Willing, B. P., Dicksved, J., Halfvarson, J., Andersson, A. F., Lucio, M., Zheng, Z., Järnerot, G., Tysk, C., Jansson, J. K., & Engstrand, L. (2010). A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology, 139, 1844–1854.
Wong, E. T., & Tergaonkar, V. (2009). Roles of NF-κB in health and disease: Mechanisms and therapeutic potential. Clinical Science, 116, 451–465.
Wong, J. M. W., de Souza, R., Kendall, C. W. C., Emam, A., & Jenkins, D. J. A. (2006). Colonic health: Fermentation and short chain fatty acids. Journal of Clinical Gastroenterology, 40, 235–243.
Xavier, R. J., & Podolsky, D. (2007). Unravelling the pathogenesis of inflammatory bowel disease. Nature, 448, 427–434.
Zocco, M. A., Verme, L. Z., Cremonini, F., Piscaglia, A. C., Nista, E. C., Candelli, M., Novi, M., Rigante, D., Cazzato, I. A., Ojetti, V., et al. (2006). Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Alimentary Pharmacology & Therapeutics, 23, 1567–1574.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2021R1I1A1A01048923) and the Bio & Medical Technology Development Program of the NRF funded by the Korean government (MSIT) (NRF-2022M3A9F3017371).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
G.K. is the founder of KoBioLabs, Inc., and S.P. is an employee of KoBioLabs, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University, Republic of Korea (approval no. SNU-160602-9-3 and SNU-170831-1).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, Wk., Min, Sg., Kwon, H. et al. Lactobacillus rhamnosus KBL2290 Ameliorates Gut Inflammation in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. J Microbiol. 61, 673–682 (2023). https://doi.org/10.1007/s12275-023-00061-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-023-00061-5