Abstract
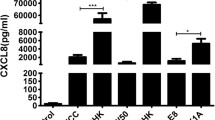
Tannerella forsythia is considered a pathogen of periodontitis and forms a biofilm with multi-species bacteria in oral cavity. Lipopolysaccharide is a powerful immunostimulator and induces inflammation and shock. The purpose of this study was to investigate the characteristics of T. forsythia LPS in its co-cultivation with Fusobacterium nucleatum or Porphyromonas gingivalis. T. forsythia was co-cultured in the presence and absence of F. nucleatum and P. gingivalis and then T. forsythia LPS was extracted. The extracts were analyzed by SDS-PAGE and NF-κB reporter CHO cell lines. THP-1 cells were treated with the LPS and evaluated induction of cytokine expression by real-time RT-PCR and ELISA. For analysis of the bioactivity of T. forsythia LPS, the binding assay on LPS-binding protein (LBP) and CD14 was processed. The extracts did not contaminate other molecules except LPS and showed TLR4 agonists. Co-cultured T. forsythia LPS with P. gingivalis exhibited a lower level of induction of TNF-α, IL-1β, and IL-6 expression than singleor co-cultured T. forsythia LPS with F. nucleatum in the conditions of human serum. However, the three T. forsythia LPS did not show difference of cytokine induction in the serum free conditions. Co-cultured T. forsythia LPS with P. gingivalis exhibited a lower affinity to LBP and CD14 as binding site of O-antigen and attached at a lower level to THP-1 cells compared to single- or co-cultured T. forsythia LPS with F. nucleatum. The virulence of T. forsythia LPS was decreased by co-culturing with P. gingivalis and their affinity to LBP and CD14 was reduced, which may due to modification of O-antigen chain by P. gingivalis.
Similar content being viewed by others
References
Chow, J.C., Young, D.W., Golenbock, D.T., Christ, W.J., and Gusovsky, F. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274, 10689–106–2.
Cooke, K.R., Olkiewicz, K., Erickson, N., and Ferrara, J.L. 2002. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J. Endotoxin Res. 8, 441–448.
Hajjar, A.M., Ernst, R.K., Tsai, J.H., Wilson, C.B., and Miller, S.I. 2002. Human toll-like receptor 4 recognizes host-specific lps modifications. Nat. Immunol. 3, 354–359.
Hojo, K., Nagaoka, S., Ohshima, T., and Maeda, N. 2009. Bacterial interactions in dental biofilm development. J. Dent. Res. 88, 982–990.
Holt, S.C. and Ebersole, J.L. 2005. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: The “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 38, 72–122.
Jack, R.S., Fan, X., Bernheiden, M., Rune, G., Ehlers, M., Weber, A., Kirsch, G., Mentel, R., Furll, B., Freudenberg, M., and et al. 1997. Lipopolysaccharide-binding protein is required to combat a murine Gram-negative bacterial infection. Nature 389, 742–745.
Jang, Y.J., Sim, J., Jun, H.K., and Choi, B.K. 2013. Differential effect of autoinducer 2 of Fusobacterium nucleatum on oral streptococci. Arch. Oral Biol. 58, 1594–1602.
Jenkinson, H.F. and Lamont, R.J. 2005. Oral microbial communities in sickness and in health. Trends Microbiol. 13, 589–595.
Kolenbrander, P.E. 2000. Oral microbial communities: Biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 54, 413–437.
Lappin, D.F., Sherrabeh, S., and Erridge, C. 2011. Stimulants of toll-like receptors 2 and 4 are elevated in saliva of periodontitis patients compared with healthy subjects. J. Clin. Periodontol. 38, 318–325.
Lee, S.H. and Baek, D.H. 2013. Characteristics of Porphyromonas gingivalis lipopolysaccharide in co-culture with Fusobacterium nucleatum. Mol. Oral Microbiol. 28, 230–238.
Lee, S.H., Jun, H.K., Lee, H.R., Chung, C.P., and Choi, B.K. 2010. Antibacterial and lipopolysaccharide (LPS)-neutralising activity of human cationic antimicrobial peptides against periodontopathogens. Int. J. Antimicrob. Agents 35, 138–145.
Lee, S.H., Kim, K.K., Rhyu, I.C., Koh, S., Lee, D.S., and Choi, B.K. 2006. Phenol/water extract of Treponema socranskii subsp. socranskii as an antagonist of toll-like receptor 4 signalling. Microbiolgy 152, 535–546.
Liljemark, W.F. and Bloomquist, C. 1996. Human oral microbial ecology and dental caries and periodontal diseases. Crit. Rev. Oral Biol. Med. 7, 180–198.
Moreno, C., Merino, J., Ramirez, N., Echeverria, A., Pastor, F., and Sanchez-Ibarrola, A. 2004. Lipopolysaccharide needs soluble CD14 to interact with TLR4 in human monocytes depleted of membrane CD14. Microbes Infect. 6, 990–995.
Morkunas, B., Galloway, W.R., Wright, M., Ibbeson, B.M., Hodgkinson, J.T., O’Connell, K.M., Bartolucci, N., Della Valle, M., Welch, M., and Spring, D.R. 2012. Inhibition of the production of the Pseudomonas aeruginosa virulence factor pyocyanin in wild-type cells by quorum sensing autoinducer-mimics. Org. Biomol. Chem. 10, 8452–8464.
Murray, J.L., Connell, J.L., Stacy, A., Turner, K.H., and Whiteley, M. 2014. Mechanisms of synergy in polymicrobial infections. J. Microbiol. 52, 188–199.
Noack, B., Genco, R.J., Trevisan, M., Grossi, S., Zambon, J.J., and De Nardin, E. 2001. Periodontal infections contribute to elevated systemic C-reactive protein level. J. Periodontol. 72, 1221–1227.
Park, S.N., Park, J.Y., and Kook, J.K. 2011. Development of Porphyromonas gingivalis-specific quantitative real-time PCR primers based on the nucleotide sequence of rpoB. J. Microbiol. 49, 315–3–9.
Posch, G., Andrukhov, O., Vinogradov, E., Lindner, B., Messner, P., Holst, O., and Schaffer, C. 2013. Structure and immunogenicity of the rough-type lipopolysaccharide from the periodontal pathogen Tannerella forsythia. Clin. Vaccine Immunol. 20, 945–953.
Raetz, C.R., Reynolds, C.M., Trent, M.S., and Bishop, R.E. 2007. Lipid a modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 76, 295–329.
Raetz, C.R. and Whitfield, C. 2002. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700.
Schroder, N.W., Heine, H., Alexander, C., Manukyan, M., Eckert, J., Hamann, L., Gobel, U.B., and Schumann, R.R. 2004. Lipopolysaccharide binding protein binds to triacylated and diacylated lipopeptides and mediates innate immune responses. J. Immunol. 173, 2683–2691.
Seo, T., Cha, S., Kim, T.I., Lee, J.S., and Woo, K.M. 2012. Porphyromonas gingivalis-derived lipopolysaccharide-mediated activation of MAPK signaling regulates inflammatory response and differentiation in human periodontal ligament fibroblasts. J. Microbiol. 50, 311–319.
Socransky, S.S. and Haffajee, A.D. 1992. The bacterial etiology of destructive periodontal disease: Current concepts. J. Periodontol. 63, 322–331.
Socransky, S.S. and Haffajee, A.D. 2005. Periodontal microbial ecology. Periodontol 2000 38, 135–187.
Socransky, S.S., Haffajee, A.D., Cugini, M.A., Smith, C., and Kent, R.L. Jr. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25, 134–144.
Yoneda, M., Hirofuji, T., Motooka, N., Nozoe, K., Shigenaga, K., Anan, H., Miura, M., Kabashima, H., Matsumoto, A., and Maeda, K. 2003. Humoral immune responses to S-layer-like proteins of Bacteroides forsythus. Clin. Diagn. Lab Immunol. 10, 383–387.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, YJ., Lee, SH. Reducing the bioactivity of Tannerella forsythia lipopolysaccharide by Porphyromonas gingivalis . J Microbiol. 52, 702–708 (2014). https://doi.org/10.1007/s12275-014-4324-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-014-4324-5