Abstract
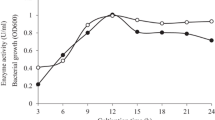
A mannanase was purified from a cell-free extract of the recombinant Escherichia coli carrying a Bacillus subtilis WL-3 mannanase gene. The molecular mass of the purified mannanase was 38 kDa as estimated by SDS-PAGE. Optimal conditions for the purified enzyme occurred at pH 6.0 and 60°C. The specific activity of the purified mannanase was 5,900 U/mg on locust bean gum (LBG) galactomannan at pH 6.0 and 50°C. The activity of the enzyme was slightly inhibited by Mg2+, Ca2+, EDTA and SDS, and noticeably enhanced by Fe2+. When the enzyme was incubated at 4°C for one day in the presence of 3 mM Fe2+, no residual activity of the mannanase was observed. The enzyme showed higher activity on LBG and konjac glucomannan than on guar gum galactomannan. Furthermore, it could hydrolyze xylans such as arabinoxylan, birchwood xylan and oat spelt xylan, while it did not exhibit any activities towards carboxymethylcellulose and para-nitrophenyl-β-mannopyranoside. The predominant products resulting from the mannanase hydrolysis were mannose, mannobiose and mannotriose for LBG or mannooligosaccharides including mannotriose, mannotetraose, mannopentaose and mannohexaose. The enzyme could hydrolyze mannooligosaccharides larger than mannobiose.
Similar content being viewed by others
References
Akino, T., N. Nakamura, and K. Horikoshi. 1988. Characterization of three β-mannanases of an alkalophilic Bacillus sp.. Agric. Biol. Chem. 52, 773–779.
Dhawan, S. and J. Kaur. 2007. Microbial mannanases: an overview of production and applications. Crit. Res. Biotechnol. 27, 197–216.
Gherardini, F.C. and A.A. Salyers. 1987. Purification and characterization of a cell-associated, soluble mannanase from Bacteroides ovatus. J. Bacteriol. 169, 2038–2043.
Gibbs, M.D., A.U. Elinder, R.A. Reeves, and P.L. Bergquist. 1996. Sequencing, cloning, and expression of a β-1,4-mannanase gene, manA, from the extremely thermophilic anaerobic bacterium, Caldicellulosiruptor Rt8B.4. FEMS Microbiol. Lett. 141, 37–43.
Gibbs, M.D., R.A. Reeves, A. Sunna, and P.L. Bergquist. 1999. Sequencing and expression of a β-mannanase gene from the extreme thermophile Dictyoglomus thermophilum Rt46B.1, and characteristics of the recombinant enzyme. Curr. Microbiol. 39, 351–357.
Hatada, Y., N. Takeda, K. Hirasawa, Y. Ohta, R. Usami, Y. Yoshida, W.D. Grant, S. Ito, and K. Horikoshi. 2005. Sequence of the gene for a high-alkaline mannanase from an alkaliphilic Bacillus sp. strain JAMB-750, its expression in Bacillus subtilis and characterization of the recombinant enzyme. Extremophiles 9, 497–500.
Hossain, M.Z., J. Abe, and S. Hizukuri. 1996. Multiple forms of β-mannanase from Bacillus sp. KK01. Enzyme Microb. Technol. 18, 95–98.
Jiang, Z., Y. Wei, D. Li, L. Li, P. Chai, and I. Kusakabe. 2006. High-level production, purification and characterization of a thermostable β-mannanase from the newly isolated Bacillus subtilis WY34. Carbohydr. Polym. 66, 68–96.
Kansoh, A.L. and Z.A. Nagieb. 2004. Xylanase and mannanase enzymes from Streptomyces galbus NR and their use in biobleaching of softwood kraft pulp. Antonie Van Leeuwenhoek 85, 103–114.
Kataoka, N. and Y. Tokiwa. 1998. Isolation and characterization of an active mannanase-producing anaerobic bacerium, Clostridium tertium KT-5A, from lotus soil. J. Appl. Microbiol. 84, 357–367.
Khanongnuch, C., K. Asada, H. Tsuruga, T. Ooi, S. Kinoshita, and S. Lumyong. 1998. β-Mannanase and xylanase of Bacillus subtilis 5H active for bleaching of crude pulp. J. Ferment. Bioeng. 86, 461–466.
Khanongnuch, C., T. Ooi, and S. Kinoshita. 1999. Cloning and nucleotide sequence of β-mannanse and cellulase genes from Bacillus sp. 5H. Wor. J. Microbiol. Biotechnol. 15, 249–258.
Kurokawa, J., E. Hemjinda, T. Arai, S. Karita, T. Kimura, K. Sakka, and K. Ohmiya. 2001. Sequence of the Clostridium thermocellum mannanase gene man26B and characterization of the translated product. Biosci. Biotechnol. Biochem. 65, 548–554.
Kweun, M.A., M.S. Lee, J.H. Choi, K.H. Cho, and K.H. Yoon. 2004. Cloning of a Bacillus subtilis WL-7 mannanase gene and characterization of the gene product. J. Microbiol. Biotechnol. 14, 1295–1302.
Ma, Y., Y. Xue, Y. Dou, Z. Xu, W. Tao, and P. Zhou. 2004. Characterization and gene cloning of a novel β-mannanase from alkaliphilic Bacillus sp. N16-5. Extremophiles 8, 447–454.
Mendoza, N.S., M. Arai, T. Kawaguchi, T. Yoshida, and L.M. Joson. 1994. Purification and properties of mannanase from Bacillus subtilis. Wor. J. Microbiol. Biotechnol. 10, 551–556.
Mendoza, N.S., M. Arai, K. Sugimoto, M. Ueda, T. Kawaguchi, and L.M. Joson. 1995. Cloning and sequencing of β-mannanase gene from Bacillus subtilis NM-39. Biochim. Biophys. Acta. 1243, 552–554.
Miller, M.L., R. Blum, W.E. Glennon, and A.L. Burton. 1960. Measurement of carboxymethylcellulase activity. Anal. Biochem. 2, 127–132.
Oda, Y., T. Komaki, and K. Tonomura. 1993. Purification and properties of extracellular β-mannanases produced by Enterococcus casseliflavus FL2121 isolated from decayed konjac. J. Ferment. Bioeng. 76, 14–18.
Stoll, D., A. Boraston, H. Stalbrand, B.W. McLean, D.G. Kilburn, and R.A.J. Warren. 2000. Mannanase Man26A from Cellulomonas fimi has a mannan-binding module. FEMS Microbiol. Lett. 183, 265–269.
Sunna, A., M.D. Gibbs, C.W.J. Chin, P.J. Nelson, and P.L. Bergquist. 1999. A gene encoding a novel mutidomain β-1,4-mannanase from Caldibacillus cellulovorans and action of the recombinant enzyme on kraft pulp. Appl. Environ. Microbiol. 66, 664–670.
Talbot, G. and J. Sygusch. 1990. Purification and characterization of thermostable β-mannanase and α-galactosidase from Bacillus stearothermophilus. Appl. Environ. Microbiol. 56, 3505–3510.
Yamaura, L., T. Matsumoto, M. Funatsu, and Y. Funatsu. 1990. Purification and some properties of endo-1,4-β-D-mannanase from Pseudomonas sp. PT-5. Agric. Biol. Chem. 54, 2425–2427.
Yoon, K.H. and B.L. Lim. 2007. Cloning and strong expression of a Bacillus subtilis WL-3 mannanase gene in B. subtilis. J. Microbiol. Biotechnol. 17, 1688–1694.
Yosida, S., Y. Sako, and A. Uchida. 1997. Purification, properties, and N-terminal amino acid sequences of guar gum-degrading enzyme from Bacillus circulans K-1. Biosci. Biotechnol. Biochem. 61, 251–255.
Zhang, J., Z.M. He, and K. Hu. 2000. Purification and characterization of β-mannanase from Bacillus licheniformis for industrial use. Biotechnol. Lett. 22, 1375–1378.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, KH., Chung, S. & Lim, BL. Characterization of the Bacillus subtilis WL-3 mannanase from a recombinant Escherichia coli . J Microbiol. 46, 344–349 (2008). https://doi.org/10.1007/s12275-008-0045-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-008-0045-y