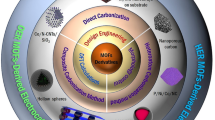
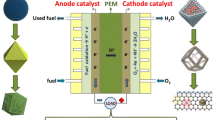
Abstract
Gas-involving electrochemical reactions, like oxygen reduction reaction (ORR), oxygen evolution reaction (OER), and hydrogen evolution reaction (HER), are critical processes for energy-saving, environment-friendly energy conversion and storage technologies which gain increasing attention. The development of according electrocatalysts is key to boost their electrocatalytic performances. Dramatic efforts have been put into the development of advanced electrocatalysts to overcome sluggish kinetics. On the other hand, the electrode interfaces-architecture construction plays an equally important role for practical applications because these imperative electrode reactions generally proceed at triple-phase interfaces of gas, liquid electrolyte, and solid electrocatalyst. A desirable architecture should facilitate the complicate reactions occur at the triple-phase interfaces, which including mass diffusion, surface reaction and electron transfer. In this review, we will summarize some design principles and synthetic strategies for optimizing triple-phase interfaces of gas-involving electrocatalysis systematically, based on the electrode reaction process at the three-phase interfaces. It can be divided into three main optimization directions: exposure of active sites, promotion of mass diffusion and acceleration of electron transfer. Furthermore, we especially highlight several remarkable works with comprehensive optimization about specific energy conversion devices, including metal-air batteries, fuel cells, and water-splitting devices are demonstrated with superb efficiency. In the last section, the perspectives and challenges in the future are proposed.

Similar content being viewed by others
References
Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303.
Chen, J. G.; Crooks, R. M.; Seefeldt, L. C.; Bren, K. L.; Bullock, R. M.; Darensbourg, M. Y.; Holland, P. L.; Hoffman, B.; Janik, M. J.; Jones, A. K. et al. Beyond fossil fuel–driven nitrogen transformations. Science 2018, 360, eaar6611.
Seh, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Norskov, J. K.; Jaramillo, T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355.
Armaroli, N.; Balzani, V. The future of energy supply: Challenges and opportunities. Angew. Chem., Int. Ed. 2007, 46, 52–66.
Wang, S. Y; Jiang, S. P. Prospects of fuel cell technologies. Natl. Sci. Rev. 2017, 4, 163–166.
Carrette, L.; Friedrich, K. A.; Stimming, U. Fuel cells–fundamentals and applications. Fuel Cells 2001, 1, 5–39.
Wang, Z. L.; Xu, D.; Xu, J. J.; Zhang, X. B. Oxygen electrocatalysts in metal-air batteries: From aqueous to nonaqueous electrolytes. Chem. Soc. Rev. 2014, 43, 7746–7786.
Cheng, F. Y.; Chen, J. Metal-air batteries: From oxygen reduction electrochemistry to cathode catalysts. Chem. Soc. Rev. 2012, 41, 2172–2192.
Kaeffer, N.; Chavarot-Kerlidou, M.; Artero, V. Hydrogen evolution catalyzed by cobalt diimine-dioxime complexes. Acc. Chem. Res. 2015, 48, 1286–1295.
Wang, J. H.; Cui, W.; Liu, Q.; Xing, Z. C.; Asiri, A. M.; Sun, X. P. Recent progress in cobalt-based heterogeneous catalysts for electrochemical water splitting. Adv. Mater. 2016, 28, 215–230.
Zhang, R. R.; Zhang, Y. C.; Pan, L.; Shen, G. Q.; Mahmood, N.; Ma, Y. H.; Shi, Y.; Jia, W. Y.; Wang, L.; Zhang, X. W. et al. Engineering cobalt defects in cobalt oxide for highly efficient electrocatalytic oxygen evolution. ACS Catal. 2018, 8, 3803–3811.
Shahraei, A.; Martinaiou, I.; Creutz, K. A.; Kübler, M.; Weidler, N.; Ranecky, S. T.; Wallace, W. D. Z.; Nowroozi, M. A.; Clemens, O.; Stark, R. W. et al. Exploring active sites in multi-heteroatom-doped Co-based catalysts for hydrogen evolution reactions. Chem.–Eur. J. 2018, 24, 12480–12484.
Katsounaros, I.; Cherevko, S.; Zeradjanin, A. R.; Mayrhofer, K. J. J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem., Int. Ed. 2014, 53, 102–121.
Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolution reaction in alkaline solutions—a review. Int. J. Hydrogen Energy 2015, 40, 256–274.
Stoerzinger, K. A.; Qiao, L.; Biegalski, M. D.; Shao-Horn, Y. Orientationdependent oxygen evolution activities of rutile IrO2 and RuO2. J. Phys. Chem. Lett. 2014, 5, 1636–1641.
Escudero-Escribano, M.; Pedersen, A. F.; Paoli, E. A.; Frydendal, R.; Friebel, D.; Malacrida, P.; Rossmeisl, J.; Stephens, I. E. L.; Chorkendorff, I. Importance of surface IrOx in stabilizing RuO2 for oxygen evolution. J. Phys. Chem. B 2018, 122, 947–955.
Tian, J. Q.; Liu, Q.; Asiri, A. M.; Sun, X. P. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590.
Sheng, W. C.; Zhuang, Z. B.; Gao, M. R.; Zheng, J.; Chen, J. G.; Yan, Y. S. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848.
Wang, Z. Q.; Ren, X.; Luo, Y. L.; Wang, L.; Cui, G. W.; Xie, F. Y.; Wang, H. J.; Xie, Y.; Sun, X. P. An ultrafine platinum-cobalt alloy decorated cobalt nanowire array with superb activity toward alkaline hydrogen evolution. Nanoscale 2018, 10, 12302–12307.
Trotochaud, L.; Ranney, J. K.; Williams, K. N.; Boettcher, S. W. Solutioncast metal oxide thin film electrocatalysts for oxygen evolution. J. Am. Chem. Soc. 2012, 134, 17253–17261.
Louie, M. W.; Bell, A. T. An investigation of thin-film Ni-Fe oxide catalysts for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337.
Zhu, Y. L.; Zhou, W.; Chen, Z. G.; Chen, Y. B.; Su, C.; Tade, M. O.; Shao, Z. P. SrNb0.1Co0.7Fe0.2O3–δ perovskite as a next-generation electrocatalyst for oxygen evolution in alkaline solution. Angew. Chem., Int. Ed. 2015, 54, 3897–3901.
Suntivich, J.; May, K. J.; Gasteiger, H. A.; Goodenough, J. B.; Shao-Horn, Y. A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 2011, 334, 1383–1385.
Jung, J. I.; Jeong, H. Y.; Lee, J. S.; Kim, M. G.; Cho, J. A bifunctional perovskite catalyst for oxygen reduction and evolution. Angew. Chem., Int. Ed. 2014, 53, 4582–4586.
Zou, X. X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180.
Kong, D. S.; Wang, H. T.; Lu, Z. Y.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900.
Cao, B. F.; Veith, G. M.; Neuefeind, J. C.; Adzic, R. R.; Khalifah, P. G. Mixed close-packed cobalt molybdenum nitrides as non-noble metal electrocatalysts for the hydrogen evolution reaction. J. Am. Chem. Soc. 2013, 135, 19186–19192.
You, B.; Jiang, N.; Sheng, M. L.; Bhushan, M. W.; Sun, Y. J. Hierarchically porous urchin-like Ni2P superstructures supported on nickel foam as efficient bifunctional electrocatalysts for overall water splitting. ACS Catal. 2016, 6, 714–721.
Wu, G.; Zelenay, P. Nanostructured nonprecious metal catalysts for oxygen reduction reaction. Acc. Chem. Res. 2013, 46, 1878–1889.
Kinoshita, K. Particle size effects for oxygen reduction on highly dispersed platinum in acid electrolytes. J. Electrochem. Soc. 1990, 137, 845–848.
Dou, S.; Dong, C. L.; Hu, Z.; Huang, Y. C.; Chen, J. L.; Tao, L.; Yan, D. F.; Chen, D. W.; Shen, S. H.; Chou, S. L. et al. Atomic-scale CoOx species in metal–organic frameworks for oxygen evolution reaction. Adv. Funct. Mater. 2017, 27, 1702546.
Kibler, L. A.; El-Aziz, A. M.; Hoyer, R.; Kolb, D. M. Tuning reaction rates by lateral strain in a palladium monolayer. Angew. Chem., Int. Ed. 2005, 44, 2080–2084.
Yang, D. S.; Chen, T.; Wang, Z. J. Electrochemical reduction of aqueous nitrogen (N2) at a low overpotential on (110)-oriented mo nanofilm. J. Mater. Chem. A 2017, 5, 18967–18971.
Xie, C.; Wang, Y. Y.; Hu, K.; Tao, L.; Huang, X. B.; Huo, J.; Wang, S. Y. In situ confined synthesis of molybdenum oxide decorated nickel–iron alloy nanosheets from MoO4 2− intercalated layered double hydroxides for the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 87–91.
Wang, Y. Y.; Zhang, Y. Q.; Liu, Z. J.; Xie, C.; Feng, S.; Liu, D. D.; Shao, M. F.; Wang, S. Y. Layered double hydroxide nanosheets with multiple vacancies obtained by dry exfoliation as highly efficient oxygen evolution electrocatalysts. Angew. Chem., Int. Ed. 2017, 56, 5867–5871.
Wang, S. Y.; Yu, D. S.; Dai, L. M. Polyelectrolyte functionalized carbon nanotubes as efficient metal-free electrocatalysts for oxygen reduction. J. Am. Chem. Soc. 2011, 133, 5182–5185.
Dou, S.; Shen, A. L.; Tao, L.; Wang, S. Y. Molecular doping of graphene as metal-free electrocatalyst for oxygen reduction reaction. Chem. Commun. 2014, 50, 10672–10675.
Wang, S. Y.; Zhang, L. P.; Xia, Z. H.; Roy, A.; Chang, D. W.; Baek, J. B.; Dai, L. M. BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2012, 124, 4285–4288.
Shen, A. L.; Zou, Y. Q.; Wang, Q.; Dryfe, R. A. W.; Huang, X. B.; Dou, S.; Dai, L. M.; Wang, S. Y. Oxygen reduction reaction in a droplet on graphite: Direct evidence that the edge is more active than the basal plane. Angew. Chem., Int. Ed. 2014, 126, 10980–10984.
Tao, L.; Qiao, M.; Jin, R.; Li, Y.; Xiao, Z. H.; Wang, Y. Q.; Zhang, N. N.; Xie, C.; He, Q. G.; Jiang, D. C. et al. Bridging the surface charge and catalytic activity of a defective carbon electrocatalyst. Angew. Chem., Int. Ed. 2019, 58, 1019–1024.
Zhang, Y. Q.; Guo, L.; Tao, L.; Lu, Y. B.; Wang, S. Y. Defect-based singleatom electrocatalysts. Small Methods 2018, 1800406.
Paraknowitsch, J. P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855.
Bondarenko, A. S.; Stephens, I. E. L.; Hansen, H. A.; Pérez-Alonso, F. J.; Tripkovic, V.; Johansson, T. P.; Rossmeisl, J.; Nørskov, J. K.; Chorkendorff, I. The Pt(111)/electrolyte interface under oxygen reduction reaction conditions: An electrochemical impedance spectroscopy study. Langmuir 2011, 27, 2058–2066.
Tang, C.; Wang, H. F.; Zhang, Q. Multiscale principles to boost reactivity in gas-involving energy electrocatalysis. Acc. Chem. Res. 2018, 51, 881–889.
Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S. Z. Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086.
Sheng, X.; Liu, Z.; Zeng, R. S.; Chen, L. P.; Feng, X. J.; Jiang, L. Enhanced photocatalytic reaction at air–liquid–solid joint interfaces. J. Am. Chem. Soc. 2017, 139, 12402–12405.
Jiao, Y.; Zheng, Y.; Davey, K.; Qiao, S. Z. Activity origin and catalyst design principles for electrocatalytic hydrogen evolution on heteroatom-doped graphene. Nat. Energy 2016, 1, 16130.
Buurmans, I. L. C.; Weckhuysen, B. M. Heterogeneities of individual catalyst particles in space and time as monitored by spectroscopy. Nat. Chem. 2012, 4, 873–886.
Shao, Y. Y.; Yin, G. P.; Wang, Z. B.; Gao, Y. Z. Proton exchange membrane fuel cell from low temperature to high temperature: Material challenges. J. Power Sources 2007, 167, 235–242.
Wei, Z. D.; Chen, S. G.; Liu, Y.; Sun, C. X.; Shao, Z. G.; Shen, P. K. Electrodepositing Pt by modulated pulse current on a nafion-bonded carbon substrate as an electrode for PEMFC. J. Phys. Chem. C 2007, 111, 15456–15463.
Zhao, Y. F.; Jia, X. D.; Chen, G. B.; Shang, L.; Waterhouse, G. I. N.; Wu, L. Z.; Tung, C. H.; O’Hare, D.; Zhang, T. R. Ultrafine NiO nanosheets stabilized by TiO2 from monolayer NiTi-LDH precursors: An active water oxidation electrocatalyst. J. Am. Chem. Soc. 2016, 138, 6517–6524.
Zhang, J. T.; Xia, Z. H.; Dai, L. M. Carbon-based electrocatalysts for advanced energy conversion and storage. Sci. Adv. 2015, 1, e1500564.
Wang, X. J.; Zhou, J. W.; Fu, H.; Li, W.; Fan, X. X.; Xin, G. B.; Zheng, J.; Li, X. G. MOF derived catalysts for electrochemical oxygen reduction. J. Mater. Chem. A 2014, 2, 14064–14070.
Ma, Z. L.; Tao, L.; Liu, D. D.; Li, Z.; Zhang, Y. Q.; Liu, Z. J.; Liu, H. W.; Chen, R.; Huo, J.; Wang, S. Y. Ultrafine nano-sulfur particles anchored on in situ exfoliated graphene for lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 9412–9417.
Yang, S. B.; Feng, X. L.; Wang, X. C.; Müllen, K. Graphene-based carbon nitride nanosheets as efficient metal-free electrocatalysts for oxygen reduction reactions. Angew. Chem. 2011, 123, 5451–5455.
Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chem., Int. Ed. 2010, 49, 8328–8344.
Dou, S.; Tao, L.; Huo, J.; Wang, S. Y.; Dai, L. M. Etched and doped Co9S8/graphene hybrid for oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 1320–1326.
Yang, M. J.; Hu, X. H.; Fang, Z. S.; Sun, L.; Yuan, Z. K.; Wang, S. Y.; Hong, W.; Chen, X. D.; Yu, D. S. Bifunctional MOF-derived carbon photonic crystal architectures for advanced Zn–air and Li–S batteries: Highly exposed graphitic nitrogen matters. Adv. Funct. Mater. 2017, 27, 1701971.
Li, K.; Li, Y.; Wang, Y. M.; Ge, J. J.; Liu, C. P.; Xing, W. Enhanced electrocatalytic performance for the hydrogen evolution reaction through surface enrichment of platinum nanoclusters alloying with ruthenium in situ embedded in carbon. Energy Environ. Sci. 2018, 11, 1232–1239.
Tian, G. L.; Zhang, Q.; Zhang, B. S.; Jin, Y. G.; Huang, J. Q.; Su, D. S.; Wei, F. Toward full exposure of “active sites”: Nanocarbon electrocatalyst with surface enriched nitrogen for superior oxygen reduction and evolution reactivity. Adv. Funct. Mater. 2014, 24, 5956–5961.
Chen, C.; Khosrowabadi Kotyk, J. F.; Sheehan, S. W. Progress toward commercial application of electrochemical carbon dioxide reduction. Chem. 2018, 4, 2571–2586.
Xu, W. W.; Lu, Z. Y.; Sun, X. M.; Jiang, L.; Duan, X. Superwetting electrodes for gas-involving electrocatalysis. Acc. Chem. Res. 2018, 51, 1590–1598.
Park, S.; Lee, J. W.; Popov, B. N. A review of gas diffusion layer in PEM fuel cells: Materials and designs. Int. J. Hydrogen Energy 2012, 37, 5850–5865.
Kreuer, K. D. Proton conductivity: Materials and applications. Chem. Mater. 1996, 8, 610–641.
Lu, Z. Y.; Xu, W. W.; Ma, J.; Li, Y. J.; Sun, X. M.; Jiang, L. Superaerophilic carbon-nanotube-array electrode for high-performance oxygen reduction reaction. Adv. Mater. 2016, 28, 7155–7161.
Li, J.; Chen, G. X.; Zhu, Y. Y.; Liang, Z.; Pei, A.; Wu, C. L.; Wang, H. X.; Lee, H. R.; Liu, K.; Chu, S. et al. Efficient electrocatalytic CO2 reduction on a three-phase interface. Nat. Catal. 2018, 1, 592–600.
Du, L.; Shao, Y. Y.; Sun, J. M.; Yin, G. P.; Liu, J.; Wang, Y. Advanced catalyst supports for PEM fuel cell cathodes. Nano Energy 2016, 29, 314–322.
Cindrella, L.; Kannan, A. M.; Lin, J. F.; Saminathan, K.; Ho, Y.; Lin, C. W.; Wertz, J. Gas diffusion layer for proton exchange membrane fuel cells— a review. J. Power Sources 2009, 194, 146–160.
Lin, G. Y.; van Nguyen, T. Effect of thickness and hydrophobic polymer content of the gas diffusion layer on electrode flooding level in a PEMFC. J. Electrochem. Soc. 2005, 152, A1942–A1948.
Snyder, J.; Fujita, T.; Chen, M. W.; Erlebacher, J. Oxygen reduction in nanoporous metal–ionic liquid composite electrocatalysts. Nat. Mater. 2010, 9, 904–907.
Snyder, J.; Livi, K.; Erlebacher, J. Oxygen reduction reaction performance of [MTBD][beti]-encapsulated nanoporous nipt alloy nanoparticles. Adv. Funct. Mater. 2013, 23, 5494–5501.
Zhang, G. R.; Munoz, M.; Etzold, B. J. M. Boosting performance of low temperature fuel cell catalysts by subtle ionic liquid modification. ACS Appl. Mater. Interfaces 2015, 7, 3562–3570.
Qiao, M.; Tang, C.; Tanase, L. C.; Teodorescu, C. M.; Chen, C. M.; Zhang, Q.; Titirici, M. M. Oxygenophilic ionic liquids promote the oxygen reduction reaction in Pt-free carbon electrocatalysts. Mater. Horiz. 2017, 4, 895–899.
Tan, Y. M.; Xu, C. F.; Chen, G. X.; Zheng, N. F.; Xie, Q. J. A graphene–platinum nanoparticles–ionic liquid composite catalyst for methanol-tolerant oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 6923–6927.
Li, W.; Liu, J.; Zhao, D. Y. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023.
Li, S. L.; Xu, Q. Metal–organic frameworks as platforms for clean energy. Energy Environ. Sci. 2013, 6, 1656–1683.
Dou, S.; Wang, X.; Wang, S. Y. Rational design of transition metal-based materials for highly efficient electrocatalysis. Small Methods 2019, 3, 1800211.
Wang, X.; Li, X. Y.; Ouyang, C. B.; Li, Z.; Dou, S.; Ma, Z. L.; Tao, L.; Huo, J.; Wang, S. Y. Nonporous MOF-derived dopant-free mesoporous carbon as an efficient metal-free electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2016, 4, 9370–9374.
Pampel, J.; Fellinger, T. P. Opening of bottleneck pores for the improvement of nitrogen doped carbon electrocatalysts. Adv. Energy Mater. 2016, 6, 1502389.
Xia, W.; Zou, R. Q.; An, L.; Xia, D. G.; Guo, S. J. A metal–organic framework route to in situ encapsulation of Co@Co3O4@C core@bishell nanoparticles into a highly ordered porous carbon matrix for oxygen reduction. Energy Environ. Sci. 2015, 8, 568–576.
Mazloomi, S. K.; Sulaiman, N. Influencing factors of water electrolysis electrical efficiency. Renew. Sust. Energ. Rev. 2012, 16, 4257–4263.
Wang, S.; Jiang, L. Definition of superhydrophobic states. Adv. Mater. 2007, 19, 3423–3424.
Lu, Z. Y.; Zhu, W.; Yu, X. Y.; Zhang, H. C.; Li, Y. J.; Sun, X. M.; Wang, X. W.; Wang, H.; Wang, J. M.; Luo, J. et al. Ultrahigh hydrogen evolution performance of under-water “superaerophobic” MoS2 nanostructured electrodes. Adv. Mater. 2014, 26, 2683–2687.
Wu, D.; Wu, S. Z.; Chen, Q. D.; Zhang, Y. L.; Yao, J.; Yao, X.; Niu, L. G.; Wang, J. N.; Jiang, L.; Sun, H. B. Curvature-driven reversible in situ switching between pinned and roll-down superhydrophobic states for water droplet transportation. Adv. Mater. 2011, 23, 545–549.
Faber, M. S.; Dziedzic, R.; Lukowski, M. A.; Kaiser, N. S.; Ding, Q.; Jin, S. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061.
Harrison, J. A.; Kuhn, A. T. The role of gas bubble formation in the electrocatalysis of the hydrogen evolution reaction. Surf. Technol. 1983, 19, 249–259.
Li, Y. J.; Zhang, H. C.; Xu, T. H.; Lu, Z. Y.; Wu, X. C.; Wan, P. B.; Sun, X. M.; Jiang, L. Under-water superaerophobic pine-shaped Pt nanoarray electrode for ultrahigh-performance hydrogen evolution. Adv. Funct. Mater. 2015, 25, 1737–1744.
He, J. L.; Hu, B. B.; Zhao, Y. Superaerophobic electrode with metal@metaloxide powder catalyst for oxygen evolution reaction. Adv. Funct. Mater. 2016, 26, 5998–6004.
Wang, Z. J.; Lu, Y. Z.; Yan, Y.; Larissa, T. Y. P.; Zhang, X.; Wuu, D.; Zhang, H.; Yang, Y. H.; Wang, X. Core-shell carbon materials derived from metalorganic frameworks as an efficient oxygen bifunctional electrocatalyst. Nano Energy 2016, 30, 368–378.
Dou, S.; Wang, X.; Wang, S. Y. Rational design of transition metal-based materials for highly efficient electrocatalysis. Small Methods 2019, 3, 1800211.
Zeng, Y. F.; Wang, Y. Y.; Huang, G.; Chen, C.; Huang, L. L.; Chen, R.; Wang, S. Y. Porous CoP nanosheets converted from layered double hydroxides with superior electrochemical activity for hydrogen evolution reactions at wide pH ranges. Chem. Commun. 2018, 54, 1465–1468.
Tao, L.; Shi, Y. L.; Huang, Y. C.; Chen, R.; Zhang, Y. Q.; Huo, J.; Zou, Y. Q.; Yu, G.; Luo, J.; Dong, C. L. et al. Interface engineering of Pt and CeO2 nanorods with unique interaction for methanol oxidation. Nano Energy 2018, 53, 604–612.
Zhong, H. X.; Wang, J.; Zhang, Y. W.; Xu, W. L.; Xing, W.; Xu, D.; Zhang, Y. F.; Zhang, X. B. ZIF-8 derived graphene-based nitrogen-doped porous carbon sheets as highly efficient and durable oxygen reduction electrocatalysts. Angew. Chem., Int. Ed. 2014, 53, 14235–14239.
Hou, Y.; Wen, Z. H.; Cui, S. M.; Ci, S. Q.; Mao, S.; Chen, J. H. An advanced nitrogen-doped graphene/cobalt-embedded porous carbon polyhedron hybrid for efficient catalysis of oxygen reduction and water splitting. Adv. Funct. Mater. 2015, 25, 872–882.
Dou, S.; Li, X. Y.; Tao, L.; Huo, J.; Wang, S. Y. Cobalt nanoparticleembedded carbon nanotube/porous carbon hybrid derived from MOFencapsulated Co3O4 for oxygen electrocatalysis. Chem. Commun. 2016, 52, 9727–9730.
Lai, J. P.; Nsabimana, A.; Luque, R.; Xu, G. B. 3D porous carbonaceous electrodes for electrocatalytic applications. Joule 2018, 2, 76–93.
Zhou, W. J.; Wu, X. J.; Cao, X. H.; Huang, X.; Tan, C. L.; Tian, J.; Liu, H.; Wang, J. Y.; Zhang, H. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energy Environ. Sci. 2013, 6, 2921–2924.
Yuan, C. Z.; Yang, L.; Hou, L. R.; Shen, L. F.; Zhang, X. G.; Lou, X. W. Growth of ultrathin mesoporous Co3O4 nanosheet arrays on Ni foam for high-performance electrochemical capacitors. Energy Environ. Sci. 2012, 5, 7883–7887.
Du, S. C.; Ren, Z. Y.; Zhang, J.; Wu, J.; Xi, W.; Zhu, J. Q.; Fu, H. G. Co3O4 nanocrystal ink printed on carbon fiber paper as a large-area electrode for electrochemical water splitting. Chem. Commun. 2015, 51, 8066–8069.
Liu, Z. J.; Zhao, Z. H.; Wang, Y. Y.; Dou, S.; Yan, D. F.; Liu, D. D.; Xia, Z. H.; Wang, S. Y. In situ exfoliated, edge-rich, oxygen-functionalized graphene from carbon fibers for oxygen electrocatalysis. Adv. Mater. 2017, 29, 1606207.
Ma, T. Y.; Dai, S.; Jaroniec, M.; Qiao, S. Z. Graphitic carbon nitride nanosheet–carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew. Chem., Int. Ed. 2014, 126, 7409–7413.
Xiu, L. Y.; Wang, Z. Y.; Yu, M. Z.; Wu, X. H.; Qiu, J. S. Aggregationresistant 3D Mxene-based architecture as efficient bifunctional electrocatalyst for overall water splitting. ACS Nano 2018, 12, 8017–8028.
Wu, Z. S.; Yang, S. B.; Sun, Y.; Parvez, K.; Feng, X. L.; Müllen, K. 3D nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient electrocatalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085.
Duan, J. J.; Chen, S.; Jaroniec, M.; Qiao, S. Z. Porous C3N4-nanolayers@N-graphene films as catalyst electrodes for highly efficient hydrogen evolution. ACS Nano 2015, 9, 931–940.
Choi, H. J.; Jung, S. M.; Seo, J. M.; Chang, D. W.; Dai, L. M.; Baek, J. B. Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 2012, 1, 534–551.
Liu, Q.; Wang, Y. B.; Dai, L. M.; Yao, J. N. Scalable fabrication of nanoporous carbon fiber films as bifunctional catalytic electrodes for flexible Zn-air batteries. Adv. Mater. 2016, 28, 3000–3006.
Hu, C. G.; Dai, L. M. Carbon-based metal-free catalysts for electrocatalysis beyond the ORR. Angew. Chem., Int. Ed. 2016, 55, 11736–11758.
Wang, Y. Q.; Tao, L.; Xiao, Z. H.; Chen, R.; Jiang, Z. Q.; Wang, S. Y. 3D carbon electrocatalysts in situ constructed by defect-rich nanosheets and polyhedrons from NaCl-sealed zeolitic imidazolate frameworks. Adv. Funct. Mater. 2018, 28, 1705356.
Ding, W.; Li, L.; Xiong, K.; Wang, Y.; Li, W.; Nie, Y.; Chen, S. G.; Qi, X. Q.; Wei, Z. D. Shape fixing via salt recrystallization: A morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2015, 137, 5414–5420.
Xu, W. W.; Lu, Z. Y.; Wan, P. B.; Kuang, Y.; Sun, X. M. High-performance water electrolysis system with double nanostructured superaerophobic electrodes. Small 2016, 12, 2492–2498.
Lee, H. K.; Koh, C. S. L.; Lee, Y. H.; Liu, C.; Phang, I. Y.; Han, X. M.; Tsung, C. K.; Ling, X. Y. Favoring the unfavored: Selective electrochemical nitrogen fixation using a reticular chemistry approach. Sci. Adv. 2018, 4, eaar3208.
Xiong, K.; Peng, L. S.; Wang, Y.; Liu, L. H.; Deng, Z. H.; Li, L.; Wei, Z. D. In situ growth of RuO2-TiO2 catalyst with flower-like morphologies on the Ti substrate as a binder-free integrated anode for chlorine evolution. J. Appl. Electrochem. 2016, 46, 841–849.
Lu, Z. Y.; Sun, M.; Xu, T. H.; Li, Y. J.; Xu, W. W.; Chang, Z.; Ding, Y.; Sun, X. M.; Jiang, L. Superaerophobic electrodes for direct hydrazine fuel cells. Adv. Mater. 2015, 27, 2361–2366.
Acknowledgements
The authors acknowledge support from the National Natural Science Foundation of China (Nos. 51402100 and 21573066) and the Provincial Natural Science Foundation of Hunan (Nos. 2016JJ1006 and 2016TP1009).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Y., Zou, Y., Tao, L. et al. Rational design of three-phase interfaces for electrocatalysis. Nano Res. 12, 2055–2066 (2019). https://doi.org/10.1007/s12274-019-2310-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2310-2