Abstract
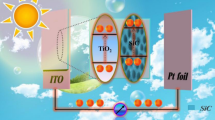
This paper presents a p–n heterojunction photoanode based on a p-type porphyrin metal–organic framework (MOF) thin film and an n-type rutile titanium dioxide nanorod array for photoelectrochemical water splitting. The TiO2@MOF core–shell nanorod array is formed by coating an 8 nm thick MOF layer on a vertically aligned TiO2 nanorod array scaffold via a layer-by-layer self-assembly method. This vertically aligned core–shell nanorod array enables a long optical path length but a short path length for extraction of photogenerated minority charge carriers (holes) from TiO2 to the electrolyte. A p–n junction is formed between TiO2 and MOF, which improves the extraction of photogenerated electrons and holes out of the TiO2 nanorods. In addition, the MOF coating significantly improves the efficiency of charge injection at the photoanode/electrolyte interface. Introduction of Co(III) into the MOF layer further enhances the charge extraction in the photoanode and improves the charge injection efficiency. As a result, the photoelectrochemical cell with the TiO2@Co-MOF nanorod array photoanode exhibits a photocurrent density of 2.93 mA/cm2 at 1.23 V (vs. RHE), which is ~ 2.7 times the photocurrent achieved with bare TiO2 nanorod array under irradiation of an unfiltered 300 W Xe lamp with an output power density of 100 mW/cm2.

Similar content being viewed by others
References
Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38.
Kang, D.; Kim, T. W.; Kubota, S. R.; Cardiel, A. C.; Cha, H. G.; Choi, K. S. Electrochemical synthesis of photoelectrodes and catalysts for use in solar water splitting. Chem. Rev. 2015, 115, 12839–12887.
Dresselhaus, M. S.; Thomas, I. L. Alternative energy technologies. Nature 2001, 414, 332–337.
Tachibana, Y.; Vayssieres, L.; Durrant, J. R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518.
Walter, M. G.; Warren, E. L.; McKone, J. R.; Boettcher, S. W.; Mi, Q. X.; Santori, E. A.; Lewis, N. S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473.
Katz, M. J.; Riha, S. C.; Jeong, N. C.; Martinson, A. B. F.; Farha, O. K.; Hupp, J. T. Toward solar fuels: Water splitting with sunlight and “rust”? Coord. Chem. Rev. 2012, 256, 2521–2529.
Mayer, M. T.; Du, C.; Wang, D. W. Hematite/sinanowire dual-absorber system for photoelectrochemical water splitting at low applied potentials. J. Am. Chem. Soc. 2012, 134, 12406–12409.
Chernomordik, B. D.; Russell, H. B.; Cvelbar, U.; Jasinski, J. B.; Kumar, V.; Deutsch, T.; Sunkara, M. K. Photoelectrochemical activity of as-grown, α-Fe2O3 nanowire array electrodes for water splitting. Nanotechnology 2012, 23, 194009.
Osterloh, F. E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320.
Zhang, Y. H.; Tang, Z. R.; Fu, X. Z.; Xu, Y. J. TiO2-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2-graphene truly different from other TiO2-carbon composite materials? ACS Nano 2010, 4, 7303–7314.
Zhou, H. C.; Long, J. R.; Yaghi, O. M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674.
Xia, W.; Mahmood, A.; Zou, R. Q.; Xu, Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866.
Wang, W.; Xu, X. M.; Zhou, W.; Shao, Z. P. Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting. Adv. Sci. 2017, 4, 1600371.
Kuc, A.; Enyashin, A.; Seifert, G. Metal-organic frameworks: Structural, energetic, electronic, and mechanical properties. J. Phys. Chem. B 2007, 111, 8179–8186.
Zhang, P.; Guan, B. Y.; Yu, L.; Lou, X. W. Facile synthesis of multi-shelled ZnS-CdS cages with enhanced photoelectrochemical performance for solar energy conversion. Chem 2018, 4, 162–173.
Zhang, H. B.; Nai, J. W.; Yu, L.; Lou, X. W. Metal-organic-framework-based materials as platforms for renewable energy and environmental applications. Joule 2017, 1, 77–107.
Wu, H. B.; Lou, X. W. Metal-organic frameworks and their derived materials for electrochemical energy storage and conversion: Promises and challenges. Sci. Adv. 2017, 3, eaap9252.
Wang, W.; Xu, X. M.; Zhou, W.; Shao, Z. P. Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting. Adv. Sci. 2017, 4: 1600371.
Zhang, L. P.; Cui, P.; Yang, H. B.; Chen, J. Z.; Xiao, F. X.; Guo, Y. Y.; Liu, Y.; Zhang, W. N.; Huo, F. W.; Liu, B. Metal–organic frameworks as promising photosensitizers for photoelectrochemical water splitting. Adv. Sci. 2016, 3, 1500243.
Dou, Y. B.; Zhou, J.; Zhou, A. W.; Li, J. R.; Nie, Z. R. Visible-light responsive MOF encapsulation of noble-metal-sensitized semiconductors for high-performance photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5, 19491–19498.
Otsuki, J. Supramolecular approach towards light-harvesting materials based on porphyrins and chlorophylls. J. Mater. Chem. A 2018, 6, 6710–6753.
Gao, W. Y.; Chrzanowski, M.; Ma, S. Q. Metal-metalloporphyrin frameworks: A resurging class of functional materials. Chem. Soc. Rev. 2014, 43, 5841–5866.
Zhao, M.; Ou, S.; Wu, C. D. Porous metal-organic frameworks for heterogeneous biomimetic catalysis. Acc. Chem. Res. 2014, 47, 1199–1207.
Huh, S.; Kim, S. J.; Kim, Y. Porphyrinic metal-organic frameworks from custom-designed porphyrins. CrystEngComm 2016, 18, 345–368.
Farha, O. K.; Shultz, A. M.; Sarjeant, A. A.; Nguyen, S. T.; Hupp, J. T. Active-site-accessible, porphyrinic metal-organic framework materials. J. Am. Chem. Soc. 2011, 133, 5652–5655.
Deria, P.; Bury, W.; Hupp, J. T.; Farha, O. K. Versatile functionalization of the NU-1000 platform by solvent-assisted ligand incorporation. Chem. Commun. 2014, 50, 1965–1968.
So, M. C.; Jin, S. Y.; Son, H. J.; Wiederrecht, G. P.; Farha, O. K.; Hupp, J. T. Layer-by-layer fabrication of oriented porous thin films based on porphyrin-containing metal-organic frameworks. J. Am. Chem. Soc. 2013, 135, 15698–15701.
Liu, B.; Aydil, E. S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990.
Li, J. T.; Hoffmann, M. W. G.; Shen, H.; Fabrega, C.; Prades, J. D.; Andreu, T.; Hernandez-Ramirez, F.; Mathur, S. Enhanced photoelectrochemical activity of an excitonic staircase in CdS@TiO2 and CdS@anatase@rutile TiO2 heterostructures. J. Mater. Chem. 2012, 22, 20472–20476.
Jiang, H. L.; Feng, D. W.; Wang, K. C.; Gu, Z. Y.; Wei, Z. W.; Chen, Y. P.; Zhou, H. C. An exceptionally stable, porphyrinic Zr metal-organic framework exhibiting pH-dependent fluorescence. J. Am. Chem. Soc. 2013, 135, 13934–13938.
Yang, H.; Zhang, S. L.; Han, L. H.; Zhang, Z.; Xue, Z.; Gao, J.; Li, Y. J.; Huang, C. H.; Yi, Y. P.; Liu, H. B. et al. High conductive two-dimensional covalent organic framework for lithium storage with large capacity. ACS Appl. Mater. Interfaces 2016, 8, 5366–5375.
Sarno, D. M.; Matienzo, L. J.; Jones, W. E. X-ray photoelectron spectroscopy as a probe of intermolecular interactions in porphyrin polymer thin films. Inorg. Chem. 2001, 40, 6308–6315.
Fidalgo-Marijuan, A.; Barandika, G.; Bazán, B.; Urtiaga, M. K.; Arriortua, M. I. Self-assembly of iron TCPP (meso-tetra(4-carboxyphenyl)porphyrin) into a chiral 2D coordination polymer. Polyhedron 2011, 30, 2711–2716.
Sonkar, P. K.; Prakash, K.; Yadav, M.; Ganesan, V.; Sankar, M.; Gupta, R.; Yadav, D. K. Co(II)-porphyrin-decorated carbon nanotubes as catalysts for oxygen reduction reactions: An approach for fuel cell improvement. J. Mater. Chem. A 2017, 5, 6263–6276.
Meng, F. K.; Li, J. T.; Cushing, S. K.; Zhi, M. J.; Wu, N. Q. Solar hydrogen generation by nanoscale p–n junction of p-type molybdenum disulfide/n-type nitrogen-doped reduced graphene oxide. J. Am. Chem. Soc. 2013, 135, 10286–10289.
Kim, T. W.; Choi, K. S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 2014, 343, 990–994.
Zhou, M.; Bao, J.; Bi, W. T.; Zeng, Y. Q.; Zhu, R.; Tao, M. S.; Xie, Y. Efficient water splitting via a heteroepitaxial BiVO4 photoelectrode decorated with Co-Pi catalysts. ChemSusChem 2012, 5, 1420–1425.
Shaban, M.; Rabia, M.; El-Sayed, A. M. A.; Ahmed, A.; Sayed, S. Photocatalytic properties of PbS/graphene oxide/polyaniline electrode for hydrogen generation. Sci. Rep. 2017, 7, 14100.
Li, J. T.; Wu, N. Q. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2015, 5, 1360–1384.
Wang, W. H.; Dong, J. Y.; Ye, X. Z.; Li, Y.; Ma, Y. R.; Qi, L. M. Heterostructured TiO2 nanorod@nanobowl arrays for efficient photoelectrochemical water splitting. Small 2016, 12, 1469–1478.
Hwang, Y. J.; Hahn, C.; Liu, B.; Yang, P. D. Photoelectrochemical properties of TiO2 nanowire arrays: A study of the dependence on length and atomic layer deposition coating. ACS Nano 2012, 6, 5060–5069.
Kronik, L.; Shapira, Y. Surface photovoltage phenomena: Theory, experiment, and applications. Surf. Sci. Rep. 1999, 37, 1–206.
Goodman, A. M. A method for the measurement of short minority carrier diffusion lengths in semiconductors. J. Appl. Phys. 1961, 32, 2550–2552.
Lagowski, J. Semiconductor surface spectroscopies: The early years. Surf. Sci. 1994, 299–300, 92–101.
Zhong, D. K.; Choi, S.; Gamelin, D. R. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W:BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377.
Yourey, J. E.; Pyper, K. J.; Kurtz, J. B.; Bartlett, B. M. Chemical stability of CuWO4 for photoelectrochemical water oxidation. J. Phys. Chem. C 2013, 117, 8708–8718.
Nakazono, T.; Parent, A. R.; Sakai, K. Cobalt porphyrins as homogeneous catalysts for water oxidation. Chem. Commun. 2013, 49, 6325–6327.
Abdi, F. F.; van de Krol, R. Nature and light dependence of bulk recombination in Co-Pi-catalyzed BiVO4 photoanodes. J. Phys. Chem. C 2012, 116, 9398–9404.
Li, J. T.; Cushing, S. K.; Zheng, P.; Senty, T.; Meng, F. K.; Bristow, A. D.; Manivannan, A.; Wu, N. Q. Solar hydrogen generation by a CdS-Au-TiO2 sandwich nanorod array enhanced with Au nanoparticle as electron relay and plasmonic photosensitizer. J. Am. Chem. Soc. 2014, 136, 8438–8449.
Li, J. T.; Cushing, S. K.; Zheng, P.; Meng, F. K.; Chu, D.; Wu, N. Q. Plasmon-induced photonic and energy-transfer enhancement of solar water splitting by a hematite nanorod array. Nat. Commun. 2013, 4, 2651.
Schmuki, P.; Böhni, H.; Bardwell, J. A. In situ characterization of anodic silicon oxide films by Ac impedance measurements. J. Electrochem. Soc. 1995, 142, 1705–1712.
Wafula, H.; Juma, A.; Sakwa, T.; Musembi, R.; Simiyu, J. A surface photovoltage study of surface defects on Co-doped TiO2 thin films deposited by spray pyrolysis. Coatings 2016, 6, 30.
Ivanov, T.; Donchev, V.; Germanova, K.; Kirilov, K. A vector model for analysing the surface photovoltage amplitude and phase spectra applied to complicated nanostructures. J. Phys. D: Appl. Phys. 2009, 42, 135302.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Yang, H., Bright, J., Kasani, S. et al. Metal–organic framework coated titanium dioxide nanorod array p–n heterojunction photoanode for solar water-splitting. Nano Res. 12, 643–650 (2019). https://doi.org/10.1007/s12274-019-2272-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-019-2272-4