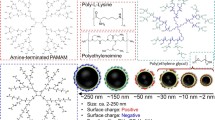
Abstract
Relationship of the surface physicochemical characteristics of nanoparticles with their interactions with biological entities may provide critical information for nanomedicinal application. Here, we report the systematic synthesis of sub-50 nm carbon nanoparticles (CNP) presenting neutral, anionic, and cationic surface functionalities. A subset of CNPs with ~10, 20, and 40 nm hydrodynamic sizes were synthesized with neutral surface headgroups. For the first time, the cellular internalization of these CNPs was systematically quantified in various stages of breast cancer cells (early, late, and metastatic), thereby providing a parametric assessment of charge and size effects. Distinct activities were observed when these systems interacted with cancer cells in various stages. Our results indicated that metastatic breast cancer could be targeted by a nanosystem presenting anionic phosphate groups. On the contrary, for patients in late stage of cancer, drugs could be delivered with sulfonate functionalized carbon nanoparticles, which have higher probability of intracellular transport. This study will facilitate the better understanding of nanoparticle–biological entity interaction, and the integration of this knowledge with pathophysiology would promote the engineering of nanomedicine with superior likelihoods of crossing the endocytic “barrier” for drug delivery inside cancerous cells.

Similar content being viewed by others
References
Anselmo, A. C.; Mitragotri, S. Nanoparticles in the clinic. Bioengineer. Translat. Med. 2016, 1, 10–29.
Eifler, A. C.; Thaxton, C. S. Nanoparticle therapeutics: FDA approval, clinical trials, regulatory pathways, and case study. In Biomedical Nanotechnology; Hurst, S. J., Ed.; Humana Press: New York,2011; pp 325–338.
Ferrari, M. Cancer nanotechnology: Opportunities and challenges. Nat. Rev. Cancer 2005, 5, 161–171.
Youan, B. B. C. Impact of nanoscience and nanotechnology on controlled drug delivery. Nanomedicine 2008, 3, 401–406.
Farokhzad, O. C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20.
Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36.
Wang, T. T.; Bai, J.; Jiang, X. E.; Nienhaus, G. U. Cellular uptake of nanoparticles by membrane penetration: A study combining confocal microscopy with FTIR spectroelectrochemistry. ACS Nano 2012, 6, 1251–1259.
Vercauteren, D.; Vandenbroucke, R. E.; Jones, A. T.; Rejman, J.; Demeester, J.; De Smedt, S. C.; Sanders, N. N.; Braeckmans, K. The use of inhibitors to study endocytic pathways of gene carriers: Optimization and pitfalls. Mol. Ther. 2010, 18, 561–569.
Chen, X. M.; Tian, F. L.; Zhang, X. R.; Wang, W. C. Internalization pathways of nanoparticles and their interaction with a vesicle. Soft Matter 2013, 9, 7592–7600.
Doherty, G. J.; McMahon, H. T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902.
Ivanov, A. I. Pharmacological inhibition of endocytic pathways: Is it specific enough to be useful? In Exocytosis and Endocytosis: Methods in Molecular Biology; Ivanov, A. I., Ed.; Humana Press: New York,2008; pp 15–33.
Conner, S. D.; Schmid, S. L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44.
Swanson, J. A.; Watts, C. Macropinocytosis. Trends Cell Biol. 1995, 5, 424–428.
Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275.
Lundqvist, M.; Stigler, J.; Elia, G.; Lynch, I.; Cedervall, T.; Dawson, K. A. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl. Acad. Sci. USA 2008, 105, 14265–14270.
Zhang, S. L.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2009, 21, 419–424.
Walkey, C. D.; Olsen, J. B.; Guo, H. B.; Emili, A.; Chan, W. C. W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2012, 134, 2139–2147.
Saha, K.; Kim, S. T.; Yan, B.; Miranda, O. R.; Alfonso, F. S.; Shlosman, D.; Rotello, V. M. Surface functionality of nanoparticles determines cellular uptake mechanisms in mammalian cells. Small 2013, 9, 300–305.
Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wi, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J. P.; Muller, S. et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2007, 2, 108–113.
Liang, M. T.; Lin, I. C.; Whittaker, M. R.; Minchin, R. F.; Monteiro, M. J.; Toth, I. Cellular uptake of densely packed polymer coatings on gold nanoparticles. ACS Nano 2010, 4, 403–413.
Arvizo, R. R.; Rana, S.; Miranda, O. R.; Bhattacharya, R.; Rotello, V. M.; Mukherjee, P. Mechanism of anti-angiogenic property of gold nanoparticles: Role of nanoparticle size and surface charge. Nanomedicine 2011, 7, 580–587.
Cho, E. C.; Xie, J. W.; Wurm, P. A.; Xia, Y. N. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009, 9, 1080–1084.
Liu, Z.; Liang, X. J. Nano-carbons as theranostics. Theranostics 2012, 2, 235–237.
Qin, W.; Ding, D.; Liu, J. Z.; Yuan, W. Z.; Hu, Y.; Liu, B.; Tang, B. Z. Biocompatible nanoparticles with aggregationinduced emission characteristics as far-red/near-infrared fluorescent bioprobes for in vitro and in vivo imaging applications. Adv. Funct. Mater. 2012, 22, 771–779.
Misra, S. K.; Chang, H. H.; Mukherjee, P.; Tiwari, S.; Ohoka, A.; Pan, D. Regulating biocompatibility of carbon spheres via defined nanoscale chemistry and a careful selection of surface functionalities. Sci. Rep. 2015, 5, 14986.
Ferrari, A. C.; Roberston, J. Raman spectroscopy of amorphous, nanostructured, diamond-like carbon, and nanodiamond. Phil. Trans. Roy. Soc. A 2004, 362, 2477–2512.
Mahou, R.; Wandrey, C. Versatile route to synthesize heterobifunctional poly(ethylene glycol) of variable functionality for subsequent pegylation. Polymers 2012, 4, 561–589.
Gliem, M.; Heupel, W.-M.; Spindler, V.; Harms, G. S.; Waschke, J. Actin reorganization contributes to loss of cell adhesion in pemphigus vulgaris. Am. J. Physiol.–Cell Physiol. 2010, 299, C606–C613.
Papakonstanti, E. A.; Stournaras, C. Cell responses regulated by early reorganization of actin cytoskeleton. FEBS Lett. 2008, 582, 2120–2127.
Allen, C.; Yu, Y. S.; Eisenberg, A.; Maysinger, D. Cellular internalization of PCL20-b-PEO44 block copolymer micelles. Biochim. Biophy. Acta 1999, 1421, 32–38.
Wang, L. M.; Liu, Y.; Li, W.; Jiang, X. M.; Ji, Y. L.; Wu, X. C.; Xu, L. G.; Qiu, Y.; Zhao, K.; Wei, T. T. et al. Selective targeting of gold nanorods at the mitochondria of cancer cells: Implications for cancer therapy. Nano Lett. 2011, 11, 772–780.
Kim, J. S.; Yoon, T. J.; Yu, K. N.; Noh, M. S.; Woo, M.; Kim, B. G.; Lee, K. H.; Sohn, B. H.; Park, S. B.; Lee, J. K., Cho, M. H. Cellular uptake of magnetic nanoparticle is mediated through energy-dependent endocytosis in A549 cells. J. Vet. Sci. 2006, 11, 772.
Wang, L. H.; Rothberg, K. G.; Anderson, R. G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993, 123, 1107–1117.
McMahon, H. T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533.
Stuart, A. D.; Brown, T. D. K. Entry of feline calicivirus is dependent on clathrin-mediated endocytosis and acidification in endosomes. J. Virol. 2006, 80, 7500–7509.
Macia, E.; Ehrlich, M.; Massol, R.; Boucrot, E.; Bruneer, C.; Kirchhausen, T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 2006, 10, 839–850.
Kirchhausen, T.; Macia, E.; Pelish, H. E. Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Method. Enzymol. 2008, 438, 77–93.
Chen, Y.; Wang, S.; Lu, X.; Zhang, H. R.; Fu, Y.; Luo, Y. Z. Cholesterol sequestration by nystatin enhances the uptake and activity of endostatin in endothelium via regulating distinct endocytic pathways. Blood 2011, 117, 6392–6403.
Sigismund, J.; Woelk, T.; Puri, C.; Maspero, E.; Tacchetti, C.; Transidico, P.; Di Fiore, P. P.; Polo, S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 2005, 102, 2760–2765.
Raghu, H.; Sodadasu, P. K.; Malla, R. R.; Gondi, C. S.; Estes, N.; Rao, J. S. Localization of uPAR and MMP-9 in lipid rafts is critical for migration, invasion and angiogenesis in human breast cancer cells. BMC Cancer 2010, 10, 647.
Zhang, L. W.; Monteiro-Riviere, N. A. Mechanisms of quantum dot nanoparticle cellular uptake. Toxicol. Sci. 2009, 110, 138–155.
Lunov, O.; Syrovets, T.; Loos, C.; Beil, J.; Delecher, M.; Tron, K.; Neinhaus, G. U.; Musyanovych, A.; Mailä nder, V.; Landfester, K. et al. Differential uptake of functionalized polystyrene nanoparticles by human macrophages and a monocytic cell line. ACS Nano 2011, 5, 1657–1669.
Gratton, S. E. A.; Ropp, P. A.; Pohlhaus, P. D.; Luft, J. C.; Madden, V. J.; Napier, M. E.; DeSimone, J. M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618.
Acknowledgements
We thank Frederick Seitz Materials Research Laboratory staffs, Roger Adams NMR Lab staffs, Carl R. Woese Institute of Genomic Biology microscopy suit staffs for their experimental and technical supports. We acknowledge Ayako Ohoka for help with the confocal studies. The funding support from the University of Illinois at Urbana-Champaign and Children’s Discovery Institute is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Srivastava, I., Misra, S.K., Ostadhossein, F. et al. Surface chemistry of carbon nanoparticles functionally select their uptake in various stages of cancer cells. Nano Res. 10, 3269–3284 (2017). https://doi.org/10.1007/s12274-017-1518-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1518-2