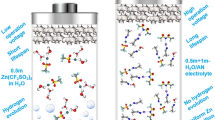
Abstract
Currently, the application of supercapacitors (SCs) in portable electronic devices and vehicles is limited by their low energy density. Developing high-energy density SCs without sacrificing their advantages, such as their long-term stability and high power density, has thus become an increasing demand but a major challenge. This demand has motivated tremendous efforts, especially towards discovering and optimizing the architecture of novel electrode materials. To this end, we herein report the design, synthesis, and SC application of a new family of two-dimensional (2D) nanoplatelets, i.e., a transition-metal hydroxymethylate complex (Ni x Co1–x (OH)(OCH3)). Bimetallic nanoplatelets were synthesized via a cost-effective approach involving a one-step solvothermal procedure. We for the first time tuned the metal composition of these 2D nanoplatelets over the entire molar-fraction range (0–1.0). Tuning the molar ratio of Ni/Co allowed us to optimize the structures and physicochemical properties of the nanoplatelets for SC applications. When tested in a half cell, SC electrodes using the nanoplatelets exhibited high electrochemical performance with a specific capacitance as high as 1,415 F·g–1 and a 96.1% retention of the initial capacitance over 5,000 cycles. We exploited the novel 2D nanoplatelets as cathode materials to assemble a hybrid SC for full-cell tests. The resulting SCs operated in a wide potential window of 0–1.7 V, exhibited a high energy density over 50 Wh·kg–1, and sustained their performance over 10,000 charge–discharge cycles. The results suggest that the novel 2D nanoplatelets are promising alternative materials for the development of high-energy density SCs.

Similar content being viewed by others
References
Winter, M.; Brodd, R. J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269.
Vlad, A.; Singh, N.; Galande, C.; Ajayan, P. M. Design considerations for unconventional electrochemical energy storage architectures. Adv. Energy Mater. 2015, 5, 1402115.
Yu, M. H.; Qiu, W. T.; Wang, F. X.; Zhai, T.; Fang, P. P.; Lu, X. H.; Tong, Y. X. Three dimensional architectures: Design, assembly and application in electrochemical capacitors. J. Mater. Chem. A 2015, 3, 15792–15823.
Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854.
Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211.
Rauda, I. E.; Augustyn, V.; Dunn, B.; Tolbert, S. H. Enhancing pseudocapacitive charge storage in polymer templated mesoporous materials. Acc. Chem. Res. 2013, 46, 1113–1124.
Yan, J.; Wang, Q.; Wei, T.; Fan, Z. J. Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv. Energy Mater. 2014, 4, 1300816.
Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614.
Zeiger, M.; Jäckel, N.; Mochalin, V. N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172–3196.
Wu, H.; Jiang, K.; Gu, S. S.; Yang, H.; Lou, Z.; Chen, D.; Shen, G. Z. Two-dimensional Ni(OH)2 nanoplates for flexible on-chip microsupercapacitors. Nano Res. 2015, 8, 3544–3552.
Park, S.; Shim, H. W.; Lee, C. W.; Song, H. J.; Park, I. J.; Kim, J. C.; Hong, K. S.; Kim, D. W. Tailoring uniform γ-MnO2 nanosheets on highly conductive three-dimensional current collectors for high-performance supercapacitor electrodes. Nano Res. 2015, 8, 990–1004.
Wang, Z.; Jia, W.; Jiang, M. L.; Chen, C.; Li, Y. D. One-step accurate synthesis of shell controllable CoFe2O4 hollow microspheres as high-performance electrode materials in supercapacitor. Nano Res. 2016, 9, 2026–2033.
Yang, M. Y.; Cheng, H.; Gu, Y. Y.; Sun, Z. F.; Hu, J.; Cao, L. J.; Lv, F. C.; Li, M. C.; Wang, W. X.; Wang, Z. Y. et al. Facile electrodeposition of 3D concentration-gradient Ni-Co hydroxide nanostructures on nickel foam as high performance electrodes for asymmetric supercapacitors. Nano Res. 2015, 8, 2744–2754.
Hu, B. L.; Qin, X. Y.; Asiri, A. M.; Alamry, K. A.; Al-Youbi, A. O.; Sun, X. P. Fabrication of Ni(OH)2 nanoflakes array on Ni foam as a binder-free electrode material for high performance supercapacitors. Electrochim. Acta 2013, 107, 339–342.
Pu, Z. H.; Liu, Q.; Qusti, A. H.; Asiri, A. M.; Al-Youbi, A. O.; Sun, X. P. Fabrication of Ni(OH)2 coated ZnO array for high-rate pseudocapacitive energy storage. Electrochim. Acta 2013, 109, 252–255.
Wang, G. P.; Zhang, L.; Zhang, J. J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828.
Liu, S.; Sun, S. H.; You, X. Z. Inorganic nanostructured materials for high performance electrochemical supercapacitors. Nanoscale 2014, 6, 2037–2045.
Yu, Z. N.; Tetard, L.; Zhai, L.; Thomas, J. Supercapacitor electrode materials: Nanostructures from 0 to 3 dimensions. Energy Environ. Sci. 2015, 8, 702–730.
Peng, X.; Peng, L. L.; Wu, C. Z.; Xie, Y. Two dimensional nanomaterials for flexible supercapacitors. Chem. Soc. Rev. 2014, 43, 3303–3323.
Kim, B. C.; Hong, J. Y.; Wallace, G. G.; Park, H. S. Recent progress in flexible electrochemical capacitors: Electrode materials, device configuration, and functions. Adv. Energy Mater. 2015, 5, 1500959.
Dong, L. B.; Xu, C. J.; Li, Y.; Huang, Z. H.; Kang, F. Y.; Yang, Q. H.; Zhao, X. Flexible electrodes and supercapacitors for wearable energy storage: A review by category. J. Mater. Chem. A 2016, 4, 4659–4685.
Peng, S. J.; Li, L. L.; Wu, H. B.; Madhavi, S.; Lou, X. W. Controlled growth of NiMoO4 nanosheet and nanorod arrays on various conductive substrates as advanced electrodes for asymmetric supercapacitors. Adv. Energy Mater. 2015, 5, 1401172.
Xu, C. J.; Shi, S.; Sun, Y. G.; Chen, Y. Y.; Kang, F. Y. Ultrathin amorphous manganese dioxide nanosheets synthesized with controllable width. Chem. Commun. 2013, 49, 7331–7333.
Hu, L. F.; Wu, L. M.; Liao, M. Y.; Hu, X. H.; Fang, X. S. Electrical transport properties of large, individual NiCo2O4 nanoplates. Adv. Funct. Mater. 2012, 22, 998–1004.
Yuan, C. Z.; Wu, H. B.; Xie, Y.; Lou, X. W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem., Int. Ed. 2014, 53, 1488–1504.
Ye, L.; Zhao, L. J.; Zhang, H.; Zhang, B.; Wang, H. Y. One-pot formation of ultra-thin Ni/Co hydroxides with a sheet-like structure for enhanced asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 9160–9168.
Chen, D.; Wang, Q. F.; Wang, R. M.; Shen, G. Z. Ternary oxide nanostructured materials for supercapacitors: A review. J. Mater. Chem. A 2015, 3, 10158–10173.
Yu, L.; Guan, B. Y.; Xiao, W.; Lou, X. W. Formation of yolk-shelled Ni-Co mixed oxide nanoprisms with enhanced electrochemical performance for hybrid supercapacitors and lithium ion batteries. Adv. Energy Mater. 2015, 5, 1500981.
Wei, T. Y.; Chen, C. H.; Chien, H. C.; Lu, S. Y.; Hu, C. C. A cost-effective supercapacitor material of ultrahigh specific capacitances: Spinel nickel cobaltite aerogels from an epoxide-driven sol-gel process. Adv. Mater. 2010, 22, 347–351.
Zhang, G. Q.; Wu, H. B.; Hoster, H. E.; Chan-Park, M. B.; Lou, X. W. Single-crystalline NiCo2O4 nanoneedle arrays grown on conductive substrates as binder-free electrodes for high-performance supercapacitors. Energy Environ. Sci. 2012, 5, 9453–9456.
Zhang, G. Q.; Lou, X. W. Controlled growth of NiCo2O4 nanorods and ultrathin nanosheets on carbon nanofibers for high-performance supercapacitors. Sci. Rep. 2013, 3, 1470.
Gao, G. X.; Wu, H. B.; Ding, S. J.; Liu, L. M.; Lou, X. W. Hierarchical NiCo2O4 nanosheets grown on Ni nanofoam as high-performance electrodes for supercapacitors. Small 2015, 11, 804–808.
Shen, L. F.; Yu, L.; Yu, X. Y.; Zhang, X. G.; Lou, X. W. Self-templated formation of uniform NiCo2O4 hollow spheres with complex interior structures for lithium-ion batteries and supercapacitors. Angew. Chem., Int. Ed. 2015, 54, 1868–1872.
Wang, C. H.; Zhang, X.; Xu, Z. T.; Sun, X. Z.; Ma, Y. W. Ethylene glycol intercalated cobalt/nickel layered double hydroxide nanosheet assemblies with ultrahigh specific capacitance: Structural design and green synthesis for advanced electrochemical storage. ACS Appl. Mater. Interfaces 2015, 7, 19601–19610.
Liu, X. X.; Zhou, A. W.; Pan, T.; Dou, Y. B.; Shao, M. F.; Han, J. B.; Wei, M. Ultrahigh-rate-capability of a layered double hydroxide supercapacitor based on a self-generated electrolyte reservoir. J. Mater. Chem. A 2016, 4, 8421–8427.
Li, T.; Li, G. H.; Li, L. H.; Liu, L.; Xu, Y.; Ding, H. Y.; Zhang, T. Large-scale self-assembly of 3D flower-like hierarchical Ni/Co-LDHs microspheres for high-performance flexible asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 2562–2572.
Gu, Y. H.; Lu, Z. Y.; Chang, Z.; Liu, J. F.; Lei, X. D.; Li, Y. P.; Sun, X. M. NiTi layered double hydroxide thin films for advanced pseudocapacitor electrodes. J. Mater. Chem. A 2013, 1, 10655–10661.
Wu, X. L.; Jiang, L. L.; Long, C. L.; Wei, T.; Fan, Z. J. Dual support system ensuring porous Co-Al hydroxide nanosheets with ultrahigh rate performance and high energy density for supercapacitors. Adv. Funct. Mater. 2015, 25, 1648–1655.
Gu, F.; Cheng, X.; Wang, S. F.; Wang, X.; Lee, P. S. Oxidative intercalation for monometallic Ni2+-Ni3+ layered double hydroxide and enhanced capacitance in exfoliated nanosheets. Small 2015, 11, 2044–2050.
Zhu, K. K.; Hu, J. C.; Kübel, C.; Richards, R. Efficient preparation and catalytic activity of MgO(111) nanosheets. Angew. Chem., Int. Ed. 2006, 45, 7277–7281.
Zhu, K. K.; Hua, W. M.; Wang, X. Y. A facile synthesis of NiO nanosheet with high-energy (111) surface. Chem. Lett. 2011, 40, 156–158.
Hutchings, G. S.; Zhang, Y.; Li, J.; Yonemoto, B. T.; Zhou, X. G.; Zhu, K. K.; Jiao, F. In situ formation of cobalt oxide nanocubanes as efficient oxygen evolution catalysts. J. Am. Chem. Soc. 2015, 137, 4223–4229.
Fan, X. L.; Liu, Z. T.; Zhu, Y.-A.; Tong, G. S.; Zhang, J. D.; Engelbrekt, C.; Ulstrup, J.; Zhu, K. K.; Zhou, X. G. Tuning the composition of metastable CoxNiyMg100-x-y(OH)(OCH3) nanoplates for optimizing robust methane dry reforming catalyst. J. Catal. 2015, 330, 106–119.
Zhu, K. K.; Hua, W. M.; Deng, W.; Richards, R. M. Preparation of MgO nanosheets with polar (111) surfaces by ligand exchange and esterification—Synthesis, structure, and application as catalyst support. Eur. J. Inorg. Chem. 2012, 2012, 2869–2876.
Liang, J. B.; Ma, R. Z.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Topochemical synthesis, anion exchange, and exfoliation of Co-Ni layered double hydroxides: A route to positively charged Co-Ni hydroxide nanosheets with tunable composition. Chem. Mater. 2010, 22, 371–378.
Han, J.; Roh, K. C.; Jo, M. R.; Kang, Y. M. A novel coprecipitation method for one-pot fabrication of a Co-Ni multiphase composite electrode and its application in high energy-density pseudocapacitors. Chem. Commun. 2013, 49, 7067–7069.
Yan, J.; Fan, Z. J.; Sun, W.; Ning, G. Q.; Wei, T.; Zhang, Q.; Zhang, R. F.; Zhi, L. J.; Wei, F. Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density. Adv. Funct. Mater. 2012, 22, 2632–2641.
Yang, J.; Yu, C.; Fan, X. M.; Qiu, J. S. 3D architecture materials made of NiCoAl-LDH nanoplates coupled with NiCo-carbonate hydroxide nanowires grown on flexible graphite paper for asymmetric supercapacitors. Adv. Energy Mater. 2014, 4, 1400761.
Wang, C. D.; Li, Y. S.; Jiang, J. J.; Chiang, W. H. Controllable tailoring graphene nanoribbons with tunable surface functionalities: An effective strategy toward highperformance lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 17441–17449.
Liu, Z. T.; Duan, X. Z.; Cheng, H. Y.; Zhou, J. H.; Zhou, X. G.; Yuan, W. K. Synthesis of platinum/graphene composites by a polyol method: The role of graphite oxide precursor surface chemistry. Carbon 2015, 89, 93–101.
Seredych, M.; Tamashausky, A. V.; Bandosz, T. J. Graphite oxides obtained from porous graphite: The role of surface chemistry and texture in ammonia retention at ambient conditions. Adv. Funct. Mater. 2010, 20, 1670–1679.
Biesinger, M. C.; Payne, B. P.; Grosvenor, A. P.; Lau, L. W. M.; Gerson, A. R.; Smart, R. S. C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730.
Yang, J.; Liu, H. W.; Martens, W. N.; Frost, R. L. Synthesis and characterization of cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 2010, 114, 111–119.
Hu, Z. A.; Xie, Y.-L.; Wang, Y.-X.; Wu, H. Y.; Yang, Y.-Y.; Zhang, Z.-Y. Synthesis and electrochemical characterization of mesoporous CoxNi1-x layered double hydroxides as electrode materials for supercapacitors. Electrochim. Acta 2009, 54, 2737–2741.
Wang, X.; Yan, C. Y.; Sumboja, A.; Yan, J.; Lee, P. S. Achieving high rate performance in layered hydroxide supercapacitor electrodes. Adv. Energy Mater. 2014, 4, 1301240.
Shen, L. F.; Yu, L.; Wu, H. B.; Yu, X. Y.; Zhang, X. G.; Lou, X. W. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat. Commun. 2015, 6, 6694.
Gao, Y.; Mi, L. W.; Wei, W. T.; Cui, S. Z.; Zheng, Z.; Hou, H. W.; Chen, W. H. Double metal ions synergistic effect in hierarchical multiple sulfide microflowers for enhanced supercapacitor performance. ACS Appl. Mater. Interfaces 2015, 7, 4311–4319.
Li, H. B.; Yu, M. H.; Wang, F. X.; Liu, P.; Liang, Y.; Xiao, J.; Wang, C. X.; Tong, Y. X.; Yang, G. W. Amorphous nickel hydroxide nanospheres with ultrahigh capacitance and energy density as electrochemical pseudocapacitor materials. Nat. Commun. 2013, 4, 1894.
Yu, Z. Y.; Cheng, Z. X.; Majid, S. R.; Tai, Z. X.; Wang, X. L.; Dou, S. X. Smart design of free-standing ultrathin Co-Co(OH)2 composite nanoflakes on 3D nickel foam for high-performance electrochemical capacitors. Chem. Commun. 2015, 51, 1689–1692.
Li, R. C.; Hu, Z. X.; Shao, X. F.; Cheng, P. P.; Li, S. S.; Yu, W. D.; Lin, W. R.; Yuan, D. S. Large scale synthesis of NiCo layered double hydroxides for superior asymmetric electrochemical capacitor. Sci. Rep. 2016, 6, 18737.
Wang, X.; Sumboja, A.; Lin, M. F.; Yan, J.; Lee, P. S. Enhancing electrochemical reaction sites in nickel-cobalt layered double hydroxides on zinc tin oxide nanowires: A hybrid material for an asymmetric supercapacitor device. Nanoscale 2012, 4, 7266–7272.
Tang, Y. F.; Liu, Y. Y.; Yu, S. X.; Guo, W. C.; Mu, S. C.; Wang, H. C.; Zhao, Y. F.; Hou, L.; Fan, Y. Q.; Gao, F. M. Template-free hydrothermal synthesis of nickel cobalt hydroxide nanoflowers with high performance for asymmetric supercapacitor. Electrochim. Acta 2015, 161, 279–289.
Sun, X.; Wang, G. K.; Sun, H. T.; Lu, F. Y.; Yu, M. P.; Lian, J. Morphology controlled high performance supercapacitor behaviour of the Ni–Co binary hydroxide system. J. Power Sources 2013, 238, 150–156.
Chen, J. C.; Hsu, C. T.; Hu, C. C. Superior capacitive performances of binary nickel–cobalt hydroxide nanonetwork prepared by cathodic deposition. J. Power Sources 2014, 253, 205–213.
Gou, J. X.; Xie, S. L.; Liu, Y. R.; Liu, C. G. Flower-like nickel-cobalt hydroxides converted from phosphites for high rate performance hybrid supercapacitor electrode materials. Electrochim. Acta 2016, 210, 915–924.
Peng, H. R.; Zhou, M.; Li, Y. H.; Cui, X.; Yang, Y.; Zhang, Y. H.; Xiao, P. Ultrahigh voltage synthesis of 2D amorphous nickel-cobalt hydroxide nanosheets on CFP for high performance energy storage device. Electrochim. Acta 2016, 190, 695–702.
Acknowledgements
This work was supported by China postdoctoral science foundation (No. 2015M580299), Fundamental Research Funds for the Central Universities (Nos. 1514011 and 222201718003), and National Natural Science Foundation of China (No. 21576082). FP7 Staff-exchange for K. K. Z. and Z. T. L. under the ELECTRONANOMAT program (No. PIRSES-GA-2012-318990) sponsored by the Marie-Curie Act program of EU is acknowledged. K. K. Z. and Z. T. L. acknowledge the Danish Agency for Science, Technology and Innovation (No. 5132-00053B) for supporting collaboration with DTU. Q. J. C. acknowledges the Danish Council for Independent Research DFF-FTP (No. 12-127447) and the Danish Agency for Science, Technology and Innovation (No. 5132-00053B) for the financial support. We thank Prof. Xinhua Zhong for helpful discussions.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
12274_2017_1517_MOESM1_ESM.pdf
New class of two-dimensional bimetallic nanoplatelets for high energy density and electrochemically stable hybrid supercapacitors
Rights and permissions
About this article
Cite this article
Liu, Z., Ma, P., Ulstrup, J. et al. New class of two-dimensional bimetallic nanoplatelets for high energy density and electrochemically stable hybrid supercapacitors. Nano Res. 10, 3018–3034 (2017). https://doi.org/10.1007/s12274-017-1517-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-017-1517-3