Abstract
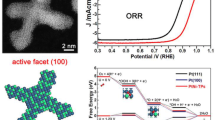
Controlled syntheses of PtNi metal nanocrystals with unique structures for catalyzing oxygen reduction reactions (ORRs) have attracted great interest. Here, we report the one-step synthesis of single-crystal PtNi octahedra with in situ-developed highly concave features and self-confined composition that are optimal for ORR. Detailed studies revealed that the Pt-rich seeding, subsequent Pt/Ni co-reduction, and Pt–Ni interfusion resulted in uniform single-crystal PtNi octahedra, and that the combination of Ni facet segregation and oxygen etching of a Ni-rich surface led to the concavity and confined Ni content. The concave PtNi nanocrystals exhibited much higher ORR performance than the commercially available Pt/C catalyst in terms of both specific activity (29.1 times higher) and mass activity (12.9 times higher) at 0.9 V (vs. reversible hydrogen electrode (RHE)). The performance was also higher than that of PtNi octahedra without concavity, confirming that the higher activity was closely related to its morphology. Moreover, the concave octahedra also exhibited remarkable stability in ORR (93% mass activity remained after 10,000 cycles between 0.6 and 1.1 V vs. RHE) owing to the passivation of the unstable sites.

Similar content being viewed by others
References
Bing, Y. H.; Liu, H. S.; Zhang, L.; Ghosh, D.; Zhang, J. J. Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction. Chem. Soc. Rev. 2010, 39, 2184–2202.
Su, D. S.; Sun, G. Q. Nonprecious-metal catalysts for low-cost fuel cells. Angew. Chem., Int. Ed. 2011, 50, 11570–11572.
Chen, Z. W.; Higgins, D.; Yu, A. P.; Zhang, L.; Zhang, J. J. A review on non-precious metal electrocatalysts for PEM fuel cells. Energy Environ. Sci. 2011, 4, 3167–3192.
Chen, J. Y.; Lim, B.; Lee, E. P.; Xia, Y. N. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95.
Wu, J. B.; Yang, H. Platinum-based oxygen reduction electrocatalysts. Acc. Chem. Res. 2013, 46, 1848–1857.
Porter, N. S.; Wu, H., Quan, Z. W.; Fang, J. Y. Shapecontrol and electrocatalytic activity-enhancement of Pt-based bimetallic nanocrystals. Acc. Chem. Res. 2013, 46, 1867–1877.
Morozan, A.; Jousselme, B.; Palacin, S. Low-platinum and platinum-free catalysts for the oxygen reduction reaction at fuel cell cathodes. Energy Environ. Sci. 2011, 4, 1238–1254.
Gasteiger, H. A.; Kocha, S. S.; Sompalli, B.; Wagner, F. T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal., B 2005, 56, 9–35.
De Bruijn, F. A.; Dam, V. A. T.; Janssen, G. J. M. Review: Durability and degradation issues of PEM fuel cell components. Fuel Cells 2008, 8, 3–22.
Greeley, J.; Stephens, I. E. L.; Bondarenko, A. S.; Johansson, T. P.; Hansen, H. A.; Jaramillo, T. F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J. K. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nat. Chem. 2009, 1, 552–556.
Gasteiger, H. A.; Markovic, N. M. Just a dream-or future reality? Science 2009, 324, 48–49.
Nørskov, J. K.; Bligaard, T.; Rossmeisl, J.; Christensen, C. H. Towards the computational design of solid catalysts. Nat. Chem. 2009, 1, 37–46.
Lee, I.; Zhang, Q.; Ge, J. P.; Yin, Y. D.; Zaera, F. Encapsulation of supported Pt nanoparticles with mesoporous silica for increased catalyst stability. Nano Res. 2011, 4, 115–123.
Li, Y. J.; Li, Y. J.; Zhu, E. B.; McLouth, T.; Chiu, C. Y.; Huang, X. Q.; Huang, Y. Stabilization of high-performance oxygen reduction reaction Pt electrocatalyst supported on reduced graphene oxide/carbon black composite. J. Am. Chem. Soc. 2012, 134, 12326–12329.
Stamenkovic, V. R.; Mun, B. S.; Arenz, M.; Mayrhofer, K. J. J.; Lucas, C. A.; Wang, G. F.; Ross, P. N.; Markovic, N. M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247.
Wu, J. B.; Gross, A.; Yang, H. Shape and compositioncontrolled platinum alloy nanocrystals using carbon monoxide as reducing agent. Nano Lett. 2011, 11, 798–802.
Zhang, J.; Fang, J. Y. A general strategy for preparation of Pt 3d-transition metal (Co, Fe, Ni) nanocubes. J. Am. Chem. Soc. 2009, 131, 18543–18547.
Chen, G. X.; Zhao, Y.; Fu, G.; Duchesne, P. N.; Gu, L.; Zheng, Y. P.; Weng, X. F.; Chen, M. S.; Zhang, P.; Pao, C.-W. et al. Interfacial effects in iron–nickel hydroxide- platinum nanoparticles enhance catalytic oxidation. Science 2014, 344, 495–499.
Stamenkovic, V. R.; Fowler, B.; Mun, B. S.; Wang, G. F.; Ross, P. N.; Lucas, C. A.; Markovic, N. M. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 2007, 315, 493–497.
Aricò, A. S.; Bruce, P.; Scrosati, B.; Tarascon, J. M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377.
Guo, S. J.; Zhang, S.; Sun, S. H. Tuning nanoparticle catalysis for the oxygen reduction reaction. Angew. Chem., Int. Ed. 2013, 52, 8526–8544.
Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y. M. et al. High-performance transition metal-doped Pt3Ni octahedra for oxygen reduction reaction. Science 2015, 348, 1230–1234.
Choi, S. I.; Xie, S. F.; Shao, M. H.; Odell, J. H.; Lu, N.; Peng, H. C.; Protsailo, L.; Guerrero, S.; Park, J.; Xia, X. H. et al. Synthesis and characterization of 9 nm Pt–Ni octahedra with a record high activity of 3.3 A/mgPt for the oxygen reduction reaction. Nano Lett. 2013, 13, 3420–3425.
Zhang, J.; Yang, H. Z.; Fang, J. Y.; Zou, S. Z. Synthesis and oxygen reduction activity of shape-controlled Pt3Ni nanopolyhedra. Nano Lett. 2010, 10, 638–644.
Huang, X. Q.; Zhu, E. B.; Chen, Y.; Li, Y. J.; Chiu, C. Y.; Xu, Y. X.; Lin, Z. Y.; Duan, X. F.; Huang, Y. A facile strategy to Pt3Ni nanocrystals with highly porous features as an enhanced oxygen reduction reaction catalyst. Adv. Mater. 2013, 25, 2974–2979.
Cui, C. H.; Gan, L.; Heggen, M.; Rudi, S.; Strasser, P. Compositional segregation in shaped Pt alloy nanoparticles and their structural behaviour during electrocatalysis. Nat. Mater. 2013, 12, 765–771.
Carpenter, M. K.; Moylan, T. E.; Kukreja, R. S.; Atwan, M. H.; Tessema, M. M. Solvothermal synthesis of platinum alloy nanoparticles for oxygen reduction electrocatalysis. J. Am. Chem. Soc. 2012, 134, 8535–8542.
Wang, X.; Choi, S. I.; Roling, L. T.; Luo, M.; Ma, C.; Zhang, L.; Chi, M. F.; Liu, J. Y.; Xie, Z. X.; Herron, J. A. et al. Palladium–platinum core–shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 2015, 6, 7594.
Zhang, L.; Roling, L. T.; Wang, X.; Vara, M.; Chi, M. F.; Liu, J. Y.; Choi, S.; Park, J.; Herron, J. A.; Xie, Z. X. et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 2015, 349, 412–416.
Li, Y. J.; Quan, F. X.; Zhu, E. B.; Chen, L.; Huang, Y.; Chen, C. F. PtxCuy nanocrystals with hexa-pod morphology and their electrocatalytic performances towards oxygen reduction reaction. Nano Res. 2015, 8, 3342–3352.
Liu, X.; Wang, W.; Li, H.; Li, L.; Zhou, G.; Yu, R.; Wang, D.; Li, Y. One-pot protocol for bimetallic Pt/Cu hexapod concave nanocrystals with enhanced electrocatalytic activity. Sci. Rep. 2013, 3, 1404.
Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H. L.; Synder, J. D.; Li, D.; Herron, J. A.; Mavrikakis, M. et al. Highly crystalline multimetallic nanoframes with threedimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343.
Li, Y. J.; Quan, F. X.; Chen, L.; Zhang, W. J.; Yu, H. B.; Chen, C. F. Synthesis of Fe-doped octahedral Pt3Ni nanocrystals with high electro-catalytic activity and stability towards oxygen reduction reaction. RSC Adv. 2014, 4, 1895–1899.
Colón-Mercado, H. R.; Popov, B. N. Stability of platinum based alloy cathode catalysts in PEM fuel cells. J. Power Sources 2006, 155, 253–263.
Kang, Y. J.; Murray, C. B. Synthesis and electrocatalytic properties of cubic Mn–Pt nanocrystals (nanocubes). J. Am. Chem. Soc. 2010, 132, 7568–7569.
Wu, Y. E.; Cai, S. F.; Wang, D. S.; He, W.; Li, Y. D. Syntheses of water-soluble octahedral, truncated octahedral, and cubic Pt–Ni nanocrystals and their structure-activity study in model hydrogenation reactions. J. Am. Chem. Soc. 2012, 134, 8975–8981.
Wang, D.; Xin, H. L.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D. A.; Di Salvo, F. J.; Abruña, H. D. Structurally ordered intermetallic platinum–cobalt core–shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87.
Wu, J. B.; Zhang, J. L.; Peng, Z. M.; Yang, S. C.; Wagner, F. T.; Yang, H. Truncated octahedral Pt3Ni oxygen reduction reaction electrocatalysts. J. Am. Chem. Soc. 2010, 132, 4984–4985.
Wu, Y. E.; Wang, D. S.; Niu, Z. Q.; Chen, P. C.; Zhou, G.; Li, Y. D. A strategy for designing a concave Pt–Ni alloy through controllable chemical etching. Angew. Chem., Int. Ed. 2012, 51, 12524–12528.
Cui, C. H.; Gan, L.; Li, H. H.; Yu, S. H.; Heggen, M.; Strasser, P. Octahedral PtNi nanoparticle catalysts: Exceptional oxygen reduction activity by tuning the alloy particle surface composition. Nano Lett. 2012, 12, 5885–5889.
Yang, H.; Vogel, W.; Lamy, C.; Alonso-Vante, N. Structure and electrocatalytic activity of carbon-supported Pt–Ni alloy nanoparticles toward the oxygen reduction reaction. J. Phys. Chem. B 2004, 108, 11024–11034.
Fortunelli, A.; Goddard III, W. A.; Sementa, L.; Barcaro, G.; Negreiros, F. R.; Jaramillo-Botero, A. The atomistic origin of the extraordinary oxygen reduction activity of Pt3Ni7 fuel cell catalysts. Chem. Sci. 2015, 6, 3915–3925.
Vitos, L.; Ruban, A. V.; Skriver, H. L.; Kollár, J. The surface energy of metals. Surf. Sci. 1998, 411, 186–202.
Gan, L.; Cui, C. H.; Heggen, M.; Dionigi, F.; Rudi, S.; Strasser, P. Element-specific anisotropic growth of shaped platinum alloy nanocrystals. Science 2014, 346, 1502–1506.
Speight, J. G. Lange's Handbook of Chemistry; McGraw-Hill: New York, 2005.
Walker, R. A.; Darby, J. B. Jr. Thermodynamic properties of solid nickel–platinum alloys. Acta Metall. 1970, 18, 1261–1266.
Wiley, B.; Sun, Y. G.; Xia, Y. N. Synthesis of silver nanostructures with controlled shapes and properties. Acc. Chem. Res. 2007, 40, 1067–1076.
Lu, Z. W.; Wei, S. H.; Zunger, A. Long-range order in binary late-transition-metal alloys. Phys. Rev. Lett. 1991, 66, 1753–1756.
Shang, S. L.; Wang, Y.; Kim, D. E.; Zacherl, C. L.; Du, Y.; Liu, Z. K. Structural, vibrational, and thermodynamic properties of ordered and disordered Ni1-xPtx alloys from first-principles calculations. Phys. Rev. B 2011, 83, 144204.
Tian, N.; Zhou, Z. Y.; Sun, S. G.; Ding, Y.; Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with highindex facets and high electro-oxidation activity. Science 2007, 316, 732–735.
Jin, M. S.; Zhang, H.; Xie, Z. X.; Xia, Y. N. Palladium concave nanocubes with high-index facets and their enhanced catalytic properties. Angew. Chem., Int. Ed. 2011, 50, 7850–7854.
Zhang, L.; Zhang, J. W.; Kuang, Q.; Xie, S. F.; Jiang, Z. Y.; Xie, Z. X.; Zheng, L. S. Cu2+-assisted synthesis of hexoctahedral Au–Pd alloy nanocrystals with high-index facets. J. Am. Chem. Soc. 2011, 133, 17114–17117.
Zhang, H.; Jin, M. S.; Xia, Y. N. Noble-metal nanocrystals with concave surfaces: Synthesis and applications. Angew. Chem., Int. Ed. 2012, 51, 7656–7673.
Zhang, J.; Langille, M. R.; Personick, M. L.; Zhang, K.; Li, S. Y.; Mirkin, C. A. Concave cubic gold nanocrystals with high-index facets. J. Am. Chem. Soc. 2012, 132, 14012–14014.
Huang, X. Q.; Zhao, Z. P.; Fan, J. M.; Tan, Y. M.; Zheng, N. F. Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets. J. Am. Chem. Soc. 2011, 133, 4718–4721.
Yu, T.; Kim, D. Y.; Zhang, H.; Xia, Y. N. Platinum concave nanocubes with high-index facets and their enhanced activity for oxygen reduction reaction. Angew. Chem., Int. Ed. 2011, 50, 2773–2777.
Xu, X. L.; Zhang, X.; Sun, H.; Yang, Y.; Dai, X. P.; Gao, J. S.; Li, X. Y.; Zhang, P. F.; Wang, H. H.; Yu, N. F. et al. Synthesis of Pt–Ni alloy nanocrystals with high-index facets and enhanced electrocatalytic properties. Angew. Chem. 2014, 126, 12730–12735.
Snyder, J.; Erlebacher, J. Kinetics of crystal etching limited by terrace dissolution. J. Electrochem. Soc. 2010, 157, C125–C130.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhu, E., Li, Y., Chiu, CY. et al. In situ development of highly concave and composition-confined PtNi octahedra with high oxygen reduction reaction activity and durability. Nano Res. 9, 149–157 (2016). https://doi.org/10.1007/s12274-015-0927-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-015-0927-3