Abstract
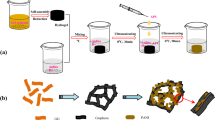
A novel morphology-controlled strategy has been developed to fabricate sulfonated graphene/polyaniline (SGEP) nanocomposites by liquid/liquid interfacial polymerization. Sulfonated graphene (SGE) sheets were synthesized and used as both a macromolecular acid dopant and substrate for the polymerization of polyaniline (PANI), affording the SGEP nanocomposites. The morphology of PANI in the nanocomposites can be controlled to be either nanorods or nanogranules by varying the synthesis conditions. The morphology of SGEP and the shape of PANI can be tuned by adding an additional dopant and varying the amount of SGE used, and this had a significant influence on the electrochemical performance of the nanocomposites as supercapacitor electrode materials. The SGEP nanocomposite with PANI nanorods exhibited a specific capacitance of 763 F/g with a capacity retention of 96% after 100 cycles and good rate properties. Composites obtained with HCl as an additional acid dopant with two different ratios of SGE to PANI showed higher specific capacitances of 793 and 931 F/g, but lower capacity retention after 100 cycles of 77% and 76%, respectively.

Similar content being viewed by others
References
Giovannetti, G.; Khomyakov, P. A.; Brocks, G.; Karpan, V. M.; Brink, J. V. D.; Kelly, P. J. Doping graphene with metal contacts. Phys. Rev. Lett. 2008, 101, 026803.
Rao, C. N. R.; Sood, A. K.; Subrahmanyam, K. S.; Govindaraj, A. Graphene: The new two-dimensional nanomaterial. Angew. Chem. Int. Ed. 2009, 48, 7752–7777.
Kou, R.; Shao, Y.; Wang, D.; Engelhard, M. H.; Kwak, J. H.; Wanga, J.; Viswanathan, V. V.; Wang, C.; Lin, Y.; Wang, Y.; Aksay, I. A.; Liu, J. Enhanced activity and stability of Pt catalysts on functionalized graphene sheets for electrocatalytic oxygen reduction. Electrochem. Commun. 2009, 11, 954–957.
Stankovich, S.; Dikin, D. A.; Dommett, G. H. B.; Kohlhaas, K. M.; Zimney, E. J.; Stach, E. A.; Piner, R. D.; Nguyen, S. T.; Ruoff, R. S. Graphene-based composite materials. Nature 2006, 442, 282–286.
Stroller, M. D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R. S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502.
Dikin, D. A.; Stankovich, S.; Zimney, E. J.; Piner, R. D.; Dommett, G. H. B.; Evmenenko, G.; Nguyen, S. T. Ruoff, R. S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460.
Muszynski, R.; Seger, B.; Kamat, P. V. Decorating graphene sheets with gold nanoparticles. J. Phys. Chem. C 2008, 112, 5263–5266.
Li, Y.; Tang, L.; Li, J. Preparation and electrochemical performance for methanol oxidation of Pt/graphene nano-composites. Electrochem. Commun. 2009, 11, 846–849.
Zhou, X.; Huang, X.; Qi, X.; Wu, S.; Xue, C. Boey, F. Y. C.; Yan, Q.; Chen, P.; Zhang, H. In situ synthesis of metal nanoparticles on single-layer graphene oxide and reduced graphene oxide surfaces. J. Phys. Chem. C 2009, 113, 10842–10846.
Si, Y.; Samulski, E. T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797.
Cao, A.; Liu, Z.; Chu, S.; Wu, M.; Ye, Z.; Cai, Z.; Chang, Y.; Wang, S.; Gong, Q.; Liu, Y. A facile one-step method to produce graphene-CdS quantum dot nanocomposites as promising optoelectronic materials. Adv. Mater. 2010, 22, 103–106.
Vickery, J. L.; Patil, A. J.; Mann, S. Fabrication of graphene-polymer nanocomposites with higher-order three-dimensional architectures. Adv. Mater. 2009, 21, 2180–2184.
Xu, Y.; Wang, Y.; Liang, J.; Huang, Y.; Ma, Y.; Wan, X.; Chen, Y. A hybrid material of graphene and poly (3,4-ethyldioxythiophene) with high conductivity, flexibility, and transparency. Nano Res. 2009, 2, 343–348.
Wang, X.; Tabakman, S. M.; Dai, H. Atomic layer deposition of metal oxides on pristine and functionalized graphene. J. Am. Chem. Soc. 2008, 130, 8152–8153.
Li, F.; Song, J.; Yang, H.; Gan, S.; Zhang, Q.; Han, D.; Ivaska, A.; Niu, L. One-step synthesis of graphene/SnO2 nanocomposites and its application in electrochemical supercapacitors. Nanotechnology 2009, 20, 455602.
Xu, C.; Wang, X.; Zhu, J.; Yang, X.; Lu, L. Deposition of Co3O4 nanoparticles onto exfoliated graphite oxide sheets. J. Mater. Chem. 2008, 18, 5625–5629.
Williams, G.; Serger, B.; Kamat, P. V. TiO2-graphene nano-composites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491.
Hao, Q.; Wang, X.; Lu, L.; Yang, X.; Mirsky, V. M. Electropolymerized multilayer conducting polymers with response to gaseous hydrogen chloride. Macromol. Rapid Commun. 2005, 26, 1099–1103.
Hao, Q.; Lei, W.; Xia, X.; Yan, Z.; Yang, X.; Lu, L.; Wang, X. Exchange of counter anions in electropolymerized polyaniline films. Electrochim. Acta 2010, 55, 632–640.
Wang D.; Li F.; Zhao J.; Ren W.; Chen Z.; Tan J.; Wu Z.; Gentle I.; Lu G., Cheng H. Fabrication of graphene/polyaniline composite paper via in situ anodic electropolymerization for high-performance flexible electrode. ACS Nano 2009, 3, 1745–1752.
Murugan, A. V.; Muraliganth, T.; Manthiram, A. Rapid, facile microwave-solvothermal synthesis of graphene nanosheets and their polyaniline nanocomposites for energy storage. Chem. Mater. 2009, 21, 5004–5006.
Wu, Q.; Xu, Y.; Yao, Z.; Liu, A.; Shi, G. Supercapacitors based on flexible graphene/polyaniline nanofiber composite films. ACS Nano 2010, 4, 1963–1970.
Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang, X. Graphene oxide doped PANI for supercapacitors. Electrochem. Commun. 2009, 11, 1158–1161.
Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang X. Effect of graphene oxide on the properties of its composite with polyaniline. ACS Appl. Mater. Interf. 2010, 2, 821–828.
Wang, H.; Hao, Q.; Yang, X.; Lu, L.; Wang X. Nanostructured graphene/polyaniline hybrid material for supercapacitors. Nanoscale, 2010, 2, 2164–2170.
Huang J.; Kaner R. B. A general chemical route to polyaniline nanofibers. J. Am. Chem. Soc., 2004, 126, 851–855
Kovtyukhova, N. I.; Ollivier, P. J.; Martin, B. R.; Mallouk, T. E.; Chizhik, S. A.; Buzaneva, E. V.; Gorchinskiy, A. D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778.
Si, Y.; Samulski, E. T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682.
Mi, H.; Zhang, X.; Yang, S.; Ye, X.; Luo, J. Polyaniline nanofibers as the electrode material for supercapacitors. Mater. Chem. Phys. 2008, 112, 127–131.
Dhand, C.; Arya, S. K.; Singh, S. P.; Singh, B. P.; Datta, M.; Malhotra, B. D. Preparation of polyaniline/multiwalled carbon nanotube composite by novel electrophoretic route. Carbon 2008, 46, 1727–1735.
Zhang, K.; Zhang, L.; Zhao, X. S.; Wu, J. Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 2010, 22, 1392–1401.
Yan, J.; Wei, T.; Shao, B.; Fan, Z.; Qian, W.; Zhang, M.; Wei, F. Preparation of a graphene nanosheet/polyaniline composite with high specific capacitance. Carbon 2010, 48, 487–493.
Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen Y. Supercapacitor devices based on graphene materials. J. Phys. Chem. C, 2009, 113, 13103–13107.
Cao, Y.; Mallouk, T. E. Morphology of template-grown polyaniline nanowires and its effect on the electrochemical capacitance of nanowire arrays. Chem. Mater. 2008, 20, 5260–5265.
Merino, C.; Soto, P.; Vilaplana-Ortego, E.; Gomez de Salazar, J. M.; Pico, F.; Rojo, J. M. Carbon nanofibres and activated carbon nanofibres as electrodes in supercapacitors. Carbon, 2005, 43, 551–557.
Liu, X.; Pickup, P. G. Ru oxide supercapacitors with high loadings and high power and energy densities. J. Power Sources, 2008, 176, 410–416.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Hao, Q., Wang, H., Yang, X. et al. Morphology-controlled fabrication of sulfonated graphene/polyaniline nanocomposites by liquid/liquid interfacial polymerization and investigation of their electrochemical properties. Nano Res. 4, 323–333 (2011). https://doi.org/10.1007/s12274-010-0087-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-010-0087-4