Abstract
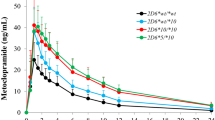
Irbesartan, a potent and selective angiotensin II type-1 (AT1) receptor blocker (ARB), is one of the representative medications for the treatment of hypertension. Cytochrome P450 (CYP) 2C9 is primarily involved in the oxidation of irbesartan. CYP2C9 is highly polymorphic, and genetic polymorphism of this enzyme is the leading cause of significant alterations in the pharmacokinetics of irbesartan. This study aimed to establish the physiologically based pharmacokinetic (PBPK) model to predict the pharmacokinetics of irbesartan in different CYP2C9 genotypes. The irbesartan PBPK model was established using the PK-Sim® software. Our previously reported pharmacogenomic data for irbesartan was leveraged in the development of the PBPK model and collected clinical pharmacokinetic data for irbesartan was used for the validation of the model. Physicochemical and ADME properties of irbesartan were obtained from previously reported data, predicted by the modeling software, or optimized to fit the observed plasma concentration–time profiles. Model evaluation was performed by comparing the predicted plasma concentration–time profiles and pharmacokinetic parameters to the observed results. Predicted plasma concentration–time profiles were visually similar to observed profiles. Predicted AUCinf in CYP2C9*1/*3 and CYP2C9*1/*13 genotypes were increased by 1.54- and 1.62-fold compared to CYP2C9*1/*1 genotype, respectively. All fold error values for AUC and Cmax in non-genotyped and CYP2C9 genotyped models were within the two-fold error criterion. We properly established the PBPK model of irbesartan in different CYP2C9 genotypes. It can be used to predict the pharmacokinetics of irbesartan for personalized pharmacotherapy in individuals of various races, ages, and CYP2C9 genotypes.





Similar content being viewed by others
References
Abduljalil K, Cain T, Humphries H, Rostami-Hodjegan (2015) Deciding on success criteria for predictability of pharmacokinetic parameters from in vitro studies: an analysis based on in vivo observations. Drug Metab Dispos 42(9):1478–1484. https://doi.org/10.1124/dmd.114.058099
Alonen A, Finel M, Kostiainen R (2008) The human UDP-glucuronosyltransferase UGT1A3 is highly selective towards N2 in the tetrazole ring of losartan, candesartan, and zolarsartan. Biochem Pharmacol 76(6):763–772. https://doi.org/10.1016/j.bcp.2008.07.006
Bae SK, Kim MJ, Shim EJ, Cho DY, Shon JH, Liu KH, Kim EY, Shin JG (2009) HPLC determination of irbesartan in human plasma: its application to pharmacokinetic studies. Biomed Chromatogr 23(6):568–572. https://doi.org/10.1002/bmc.1154
Bae JW, Choi CI, Jang CG, Lee SY (2011a) Effects of CYP2C9*1/*13 on the pharmacokinetics and pharmacodynamics of meloxicam. Br J Clin Pharmacol 71(4):550–555. https://doi.org/10.1111/j.1365-2125.2010.03853.x
Bae JW, Choi CI, Kim MJ, Oh DH, Keum SK, Park JI, Kim BH, Bang HK, Oh SG, Kang BS, Park HJ, Kim HD, Ha JH, Shin HJ, Kim YH, Na HS, Chung MW, Jang CG, Lee SY (2011b) Frequency of CYP2C9 alleles in Koreans and their effects on losartan pharmacokinetics. Acta Pharmacol Sin 32(10):1303–1308. https://doi.org/10.1038/aps.2011.100
Bae JW, Choi CI, Lee HI, Lee YJ, Jang CG, Lee SY (2012) Effects of CYP2C9*1/*3 and *1/*13 on the pharmacokinetics of losartan and its active metabolite E-3174. Int J Clin Pharmacol Ther 50(9):683–689. https://doi.org/10.5414/cp201467
Bae JW, Oh KY, Yoon SJ, Shin HB, Jung EH, Cho CK, Lim CW, Kang P, Choi CI, Jang CG, Lee SY, Lee YJ (2020) Effects of CYP2D6 genetic polymorphism on the pharmacokinetics of metoclopramide. Arch Pharm Res 43(11):1207–1213. https://doi.org/10.1007/s12272-020-01293-4
Bourrié M, Meunier V, Berger Y, Fabre G (1999) Role of cytochrome P-4502C9 in irbesartan oxidation by human liver microsomes. Drug Metab Dispos 27(2):288–296
Brunner HR (1997) The new angiotensin II receptor antagonist, irbesartan: pharmacokinetic and pharmacodynamic considerations. Am J Hypertens 10(S9):311S-317S. https://doi.org/10.1016/S0895-7061(97)00391-9
Byeon JY, Lee CM, Lee YJ, Kim YH, Kim SH, Jung EH, Chae WK, Lee YJ, Jang CG, Lee SY (2019) Influence of CYP2D6 genetic polymorphism on pharmacokinetics of active moiety of tolterodine. Arch Pharm Res 42(2):182–190. https://doi.org/10.1007/s12272-018-1099-y
Byeon JY, Cho CK, Kang P, Kim SH, Jang CG, Lee SY, Lee YJ (2023) Effects of CYP2D6 and CYP2C19 genetic polymorphisms and cigarette smoking on the pharmacokinetics of tolperisone. Arch Pharm Res 46(8):713–721. https://doi.org/10.1007/s12272-023-01462-1
Cagigal E, Gonzalez L, Alonso RM, Jiménez RM (2001) pKa determination of angiotensin II receptor antagonists (ARA II) by spectrofluorimetry. J Pharm Biomed Anal 26(3):477–486. https://doi.org/10.1016/s0731-7085(01)00413-7
Chando TJ, Everett DW, Kahle AD, Starrett AM, Vachharajani N, Shyu WC, Kripalani KJ, Barbhaiya RH (1998) Biotransformation of irbesartan in man. Drug Metab Dispos 26(5):408–417
Chapman AB, Schwartz GL, Boerwinkle E, Turner ST (2002) Predictors of antihypertensive response to a standard dose of hydrochlorothiazide for essential hypertension. Kidney Int 61(3):1047–1055. https://doi.org/10.1046/j.1523-1755.2002.00200.x
Chapy H, Klieber S, Brun P, Gerbal-Chaloin S, Boulenc X, Nicolas O (2015) PBPK modeling of irbesartan: incorporation of hepatic uptake. Biopharm Drug Dispos 36(8):491–506. https://doi.org/10.1002/bdd.1961
Chen G, Jiang S, Mao G, Zhang S, Hong X, Tang G, Li Z, Liu X, Zhang Y, Xing H, Wang B, Yu Y, Xu X (2006) CYP2C9 Ile359Leu polymorphism, plasma irbesartan concentration and acute blood pressure reductions in response to irbesartan treatment in Chinese hypertensive patients. Methods Find Exp Clin Pharmacol 28(1):19–24. https://doi.org/10.1358/mf.2006.28.1.962773
Cho CK, Kang P, Park HJ, Lee YJ, Bae JW, Jang CG, Lee SY (2021a) Physiologically based pharmacokinetic (PBPK) modelling of tamsulosin related to CYP2D6*10 allele. Arch Pharm Res 44(11):1037–1049. https://doi.org/10.1007/s12272-021-01357-z
Cho CK, Park HJ, Kang P, Moon S, Lee YJ, Bae JW, Jang CG, Lee SY (2021b) Physiologically based pharmacokinetic (PBPK) modeling of meloxicam in different CYP2C9 genotypes. Arch Pharm Res 44(12):1076–1090. https://doi.org/10.1007/s12272-021-01361-3
Cho CK, Kang P, Park HJ, Ko E, Mu CY, Lee YJ, Choi CI, Kim HS, Jang CG, Bae JW, Lee SY (2022) Physiologically based pharmacokinetic (PBPK) modeling of piroxicam with regard to CYP2C9 genetic polymorphism. Arch Pharm Res 45(5):352–366. https://doi.org/10.1007/s12272-022-01388-0
Cho CK, Byeon JY, Kang P, Park JI, Jang CG, Lee SY, Choi CI, Bae JW, Lee YJ (2023a) Effects of CYP2D6*10 allele on the pharmacokinetics of tolperisone. Arch Pharm Res 46(1):59–64. https://doi.org/10.1007/s12272-022-01422-1
Cho CK, Byeon JY, Kang P, Park HJ, Ko E, Mu CY, Jang CG, Lee SY, Lee YJ (2023b) Effects of CYP2C19 genetic polymorphism on the pharmacokinetics of tolperisone in healthy subjects. Arch Pharm Res 46(2):111–116. https://doi.org/10.1007/s12272-022-01423-0
Choi CI, Kim MJ, Jang CG, Park YS, Bae JW, Lee SY (2011) Effects of the CYP2C9*1/*13 genotype on the pharmacokinetics of lornoxicam. Basic Clin Pharmacol Toxicol 109(6):476–480. https://doi.org/10.1111/j.1742-7843.2011.00751.x
Choi CI, Kim MJ, Chung EK, Lee HI, Jang CG, Bae JW, Lee SY (2012) CYP2C9 *3 and *13 alleles significantly affect the pharmacokinetics of irbesartan in healthy Korean subjects. Eur J Clin Pharmacol 68(2):149–154. https://doi.org/10.1007/s00228-011-1098-0
Choi HY, Lim HS, Kim YH, Jeon HS, Kim MJ, Lee SH, Jung JH, Lee YK, Kim HJ, Bae KS (2015) Evaluation of the pharmacokinetics of the DPP-4 inhibitor gemigliptin when coadministered with rosuvastatin or irbesartan to healthy subjects. Curr Med Res Opin 31(2):229–241. https://doi.org/10.1185/03007995.2014.980886
Cooper-DeHoff RM, Johnson JA (2016) Hypertension pharmacogenomics: in search of personalized treatment approaches. Nat Rev Nephrol 12(2):110–122. https://doi.org/10.1038/nrneph.2015.176
Daly AK, Rettie AE, Fowler DM, Miners JO (2017) Pharmacogenomics of CYP2C9: functional and clinical considerations. J Pers Med 8(1):1. https://doi.org/10.3390/jpm8010001
Davi H, Tronquet C, Miscoria G, Perrier L, DuPont P, Caix J, Simiand J, Berger Y (2000) Disposition of irbesartan, an angiotensin II AT1-receptor antagonist, in mice, rats, rabbits, and macaques. Drug Metab Dispos 28(1):79–88
El-Desoky HS, Ghoneim MM, Habazy A (2011) Voltammetry of irbesartan drug in pharmaceutical formulations and human blood: quantification and pharmacokinetic studies. J Braz Chem Soc 22(2):239–247. https://doi.org/10.1590/S0103-50532011000200008
European Medicines Agency (2023) Aprovel: EPAR - Product Information. https://www.ema.europa.eu/en/documents/product-information/aprovel-epar-product-information_en.pdf. Accessed 06 Nov 2023
Gillis JC, Markham A (1997) Irbesartan. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in the management of hypertension. Drugs 54(6):885–902. https://doi.org/10.2165/00003495-199754060-00007
Guo Y, Zhang Y, Wang Y, Chen X, Si D, Zhong D, Fawcett JP, Zhou H (2005) Role of CYP2C9 and its variants (CYP2C9*3 and CYP2C9*13) in the metabolism of lornoxicam in humans. Drug Metab Dispos 33(6):749–753. https://doi.org/10.1124/dmd.105.003616
Hallberg P, Karlsson J, Kurland L, Lind L, Kahan T, Malmqvist K, Öhman KP, Nyström F, Melhus H (2002) The CYP2C9 genotype predicts the blood pressure response to irbesartan: results from the Swedish Irbesartan Left Ventricular Hypertrophy Investigation vs Atenolol (SILVHIA) trial. J Hypertens 20(10):2089–2093. https://doi.org/10.1097/00004872-200210000-00030
Hiltunen TP, Suonsyrjä T, Hannila-Handelberg T, Paavonen KJ, Miettinen HE, Strandberg T, Tikkanen I, Tilvis R, Pentikäinen PJ, Virolainen J, Kontula K (2007) Predictors of antihypertensive drug responses: initial data from a placebo-controlled, randomized, cross-over study with four antihypertensive drugs (The GENRES Study). Am J Hypertens 20(3):311–318. https://doi.org/10.1016/j.amjhyper.2006.09.006
Hindmarsh AC, Reynolds DR, Serban R, Woodward CS, Gardner, DJ, Cohen SD, Taylor A, Peles S, Banks L, Shumaker D (2023) Open Systems Pharmacology Suite Manual version 11 update 1. https://docs.open-systems-pharmacology.org/copyright. Accessed 06 Nov 2023
Hirvensalo P, Tornio A, Launiainen T, Paile-Hyvärinen M, Tapaninen T, Neuvonen M, Backman JT, Niemi M (2020) UGT1A3 and sex are major determinants of telmisartan pharmacokinetics—a comprehensive pharmacogenomic study. Clin Pharmacol Ther 108(4):885–895. https://doi.org/10.1002/cpt.1928
Hong X, Zhang S, Mao G, Jiang S, Zhang Y, Yu Y, Tang G, Xing H, Xu X (2005) CYP2C9* 3 allelic variant is associated with metabolism of irbesartan in Chinese population. Eur J Clin Phamracol 61(9):627–634. https://doi.org/10.1007/s00228-005-0976-8
Huang XH, Li J, Qiu FR, Xie HT, Huang JH, Li JC, Zheng QS (2006) PK-PD modeling of irbesartan in healthy Chinese adult volunteers under non-steady-state conditions. Eur J Drug Metab Pharmacokinet 31(4):259–264. https://doi.org/10.1007/BF03190465
Izumi S, Nozaki Y, Kusuhara H, Hotta K, Mochizuki T, Komori T, Maeda K, Sugiyama Y (2018) Relative activity factor (RAF)-based scaling of uptake clearance mediated by organic anion transporting polypeptide (OATP) 1B1 and OATP1B3 in human hepatocytes. Mol Pharm 15(6):2277–2288. https://doi.org/10.1021/acs.molpharmaceut.8b00138
Jeong SH, Jang JH, Lee YB (2023) P-glycoprotein mechanical functional analysis using in silico molecular modeling: Pharmacokinetic variability according to ABCB1 c.2677G > T/A genetic polymorphisms. Int J Biol Macromol 249:126777. https://doi.org/10.1016/j.ijbiomac.2023.126777
Jung EH, Lee YJ, Kim DH, Kang P, Lim CW, Cho CK, Jang CG, Lee SY, Bae JW (2020) Effects of paroxetine on the pharmacokinetics of atomoxetine and its metabolites in different CYP2D6 genotypes. Arch Pharm Res 43(12):1356–1363. https://doi.org/10.1007/s12272-020-01300-8
Jung EH, Cho CK, Kang P, Park HJ, Lee YJ, Bae JW, Choi CI, Jang CG, Lee SY (2021) Physiologically based pharmacokinetic modeling of candesartan related to CYP2C9 genetic polymorphism in adult and pediatric patients. Arch Pharm Res 44(12):1109–1119. https://doi.org/10.1007/s12272-021-01363-1
Kang P, Cho CK, Jang CG, Lee SY, Lee YJ, Choi CI, Bae JW (2023) Effects of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of gliclazide in healthy subjects. Arch Pharm Res 46(5):438–447. https://doi.org/10.1007/s12272-023-01448-z
Karatza E, Karalis V (2020) Delay differential equations for the description of Irbesartan pharmacokinetics: A population approach to model absorption complexities leading to dual peaks. Eur J Pharm Sci 153:105498. https://doi.org/10.1016/j.ejps.2020.105498
Kaur N, Thakur PS, Shete G, Gangwal R, Sangamwar AT, Bansal AK (2020) Understanding the oral absorption of irbesartan using biorelevant dissolution testing and PBPK modeling. AAPS PharmSciTech 21(3):1–13. https://doi.org/10.1208/s12249-020-01643-x
Khullar P, Kolhe V, Kulkarni A, Patel S, Phadke Y, Saravanan D, Shingte M (2015) Solid pharmaceutical fixed dose compositions comprising irbesartan and amlodipine, their preparation and their therapeutic application. United States Patent No. US009173848B2. https://patentimages.storage.googleapis.com/90/e0/b6/0df075f6d20b3a/US9173848.pdf. Accessed 06 Nov 2023
Kim SH, Kim DH, Byeon JY, Kim YH, Kim DH, Lim HJ, Lee CM, Whang SS, Choi CI, Bae JW, Lee YJ, Jang CG, Lee SY (2017) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of celecoxib and its carboxylic acid metabolite. Arch Pharm Res 40(3):382–390. https://doi.org/10.1007/s12272-016-0861-2
Kim SH, Byeon JY, Kim YH, Lee CM, Lee YJ, Jang CG, Lee SY (2018) Physiologically based pharmacokinetic modelling of atomoxetine with regard to CYP2D6 genotypes. Sci Rep 8(1):12405. https://doi.org/10.1038/s41598-018-30841-8
Kim YH, Kang P, Cho CK, Jung EH, Park HJ, Lee YJ, Bae JW, Jang CG, Lee SY (2021) Physiologically based pharmacokinetic (PBPK) modeling for prediction of celecoxib pharmacokinetics according to CYP2C9 genetic polymorphism. Arch Pharm Res 44(7):713–724. https://doi.org/10.1007/s12272-021-01346-2
Kim NT, Cho CK, Kang P, Park HJ, Lee YJ, Bae JW, Jang CG, Lee SY (2022) Effects of CYP2C9*3 and *13 alleles on the pharmacokinetics and pharmacodynamics of glipizide in healthy Korean subjects. Arch Pharm Res 45(2):114–121. https://doi.org/10.1007/s12272-021-01366-y
Kjeldsen SE (2018) Hypertension and cardiovascular risk: general aspects. Pharmacol Res 29:95–99. https://doi.org/10.1016/j.phrs.2017.11.003
Klingensmith WC, Rhea KL, Wainwright EA, Hopper OW (2010) The gastric emptying study with oatmeal: reference range and reproducibility as a function of age and sex. J Nucl Med Technol 38(4):186–190. https://doi.org/10.2967/jnmt.110.077065
Kuepfer L, Niederalt C, Wendl T, Schlender JF, Willmann S, Lippert J, Block M, Eissing T, Teutonico D (2016) Applied concepts in PBPK modeling: how to build a PBPK/PD model. CPT Pharmacomet Syst Pharmacol 5(10):516–531. https://doi.org/10.1002/psp4.12134
Lee CR, Pieper JA, Hinderliter AL, Blaisdell JA, Goldstein JA (2003) Losartan and E3174 pharmacokinetics in cytochrome P450 2C9*1/*1, *1/*2, and *1/*3 individuals. Pharmacotherapy 23(6):720–725. https://doi.org/10.1592/phco.23.6.720.32187
Lee HJ, Kim YH, Kim SH, Lee CM, Yang AY, Jang CG, Lee SY, Bae JW, Choi CI (2016) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of zafirlukast. Arch Pharm Res 39(7):1013–1019. https://doi.org/10.1007/s12272-016-0785-x
Lee HI, Byeon JY, Kim YH, Lee CM, Choi CI, Jang CG, Bae JW, Lee YJ, Lee SY (2018) Effects of CYP2C19 and CYP3A5 genetic polymorphisms on the pharmacokinetics of cilostazol and its active metabolites. Eur J Clin Pharmacol 74(11):1417–1426. https://doi.org/10.1007/s00228-018-2522-5
Lee CM, Jung EH, Byeon JY, Kim SH, Jang CG, Lee YJ, Lee SY (2019) Effects of steady-state clarithromycin on the pharmacokinetics of zolpidem in healthy subjects. Arch Pharm Res 42(12):1101–1106. https://doi.org/10.1007/s12272-019-01201-5
Lee CM, Kang P, Cho CK, Park HJ, Lee YJ, Bae JW, Choi CI, Kim HS, Jang CG, Lee SY (2022) Physiologically based pharmacokinetic modelling to predict the pharmacokinetics of metoprolol in different CYP2D6 genotypes. Arch Pharm Res. https://doi.org/10.1007/s12272-022-01394-2
Magadmi R, Alyoubi R, Moshrif T, Bakhshwin D, Suliman BA, Kamel F, Jamal M, Burzangi AS, Basit S (2023) Polymorphisms in the drug transporter gene ABCB1 are associated with drug response in Saudi epileptic pediatric patients. Biomedicines 11(9):2505. https://doi.org/10.3390/biomedicines11092505
Mann JFE, Flack JM (2023) Choice of drug therapy in primary (essential) hypertension. https://www.uptodate.com/contents/choice-of-drug-therapy-in-primary-essential-hypertension#!. Accessed 06 Nov 2023
Marino MR, Vachharajani NN (2001) Drug interactions with irbesartan. Clin Pharmacokinet 40(8):605–614. https://doi.org/10.2165/00003088-200140080-00004
Marino MR, Langenbacher K, Ford NF, Uderman HD (1998a) Pharmacokinetics and pharmacodynamics of irbesartan in healthy subjects. J Clin Pharmacol 38(3):246–255. https://doi.org/10.1002/j.1552-4604.1998.tb04422.x
Marino MR, Langenbacher KM, Raymond RH, Ford NF, Lasseter KC (1998b) Pharmacokinetics and pharmacodynamics of irbesartan in patients with hepatic cirrhosis. J Clin Pharmacol 38(4):347–356. https://doi.org/10.1002/j.1552-4604.1998.tb04434.x
Marsousi N, Desmeules JA, Rudaz S, Daali Y (2017) Usefulness of PBPK modeling in incorporation of clinical conditions in personalized medicine. J Pharm Sci 106(9):2380–2391. https://doi.org/10.1016/j.xphs.2017.04.035
McFeely SJ, Ritchie TK, Yu J, Nordmark A, Levy RH, Ragueneau-Majlessi I (2019) Identification and evaluation of clinical substrates of organic anion transporting polypeptides 1B1 and 1B3. Clin Transl Sci 12(4):379–387. https://doi.org/10.1111/cts.12623
Min JS, Bae SK (2017) Prediction of drug-drug interaction potential using physiologically based pharmacokinetic modeling. Arch Pharm Res 40(12):1356–1379. https://doi.org/10.1007/s12272-017-0976-0
Nakanishi T, Tamai I (2012) Genetic polymorphisms of OATP transporters and their impact on intestinal absorption and hepatic disposition of drugs. Drug Metab Pharmacokinet 27(1):106–121. https://doi.org/10.2133/dmpk.dmpk-11-rv-099
NCD Risk Factor Collaboration (NCD-RisC) (2021) Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. The Lancet 398(10304):957–980. https://doi.org/10.1016/S0140-6736(21)01330-1
Nishimura M, Naito S (2005) Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet 20(6):452–477. https://doi.org/10.2133/dmpk.20.452
Nishimura M, Naito S (2006) Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet 21(5):357–374. https://doi.org/10.2133/dmpk.21.357
Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T (2003) Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessedby high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi 123(5):369–375. https://doi.org/10.1248/yakushi.123.369
Oliveira-Paula GH, Pereira SC, Tanus-Santos JE, Lacchini R (2019) Pharmacogenomics and hypertension: current insights. Pharmgenomics Pers Med 12:341–359. https://doi.org/10.2147/PGPM.S230201
Perini JA, Vianna-Jorge R, Brogliato AR, Suarez-Kurtz G (2005) Influence of CYP2C9 genotypes on the pharmacokinetics and pharmacodynamics of piroxicam. Clin Pharmacol Ther 78(4):362–369. https://doi.org/10.1016/j.clpt.2005.06.014
Perrier L, Bourrié M, Marti E, Tronquet C, Massé D, Berger Y, Magdalou J, Fabre G (1994) In vitro N-glucuronidation of SB 47436 (BMS 186295), a new AT1 nonpeptide angiotensin II receptor antagonist, by rat, monkey and human hepatic microsomal fractions. J Pharmacol Exp Ther 271(1):91–99
Poulin P, Schoenlein K, Theil FP (2001) Prediction of adipose tissue: plasma partition coefficients for structurally unrelated drugs. J Pharm Sci 90(4):436–447. https://doi.org/10.1002/1520-6017(200104)90:4<436::aid-jps1002>3.0.co;2-p
Poulin P, Theil FP (2000) A priori prediction of tissue: plasma partition coefficients of drugs to facilitate the use of physiologically-based pharmacokinetic models in drug discovery. J Pharm Sci 89(1):16–35. https://doi.org/10.1002/(SICI)1520-6017(200001)89:1%3c16::AID-JPS3%3e3.0.CO;2-E
Poulin P, Theil FP (2002a) Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J Pharm Sci 91(1):129–156. https://doi.org/10.1002/jps.10005
Poulin P, Theil FP (2002b) Prediction of pharmacokinetics prior to in vivo studies. II. Generic physiologically based pharmacokinetic models of drug disposition. J Pharm Sci 91(5):1358–1370. https://doi.org/10.1002/jps.10128
Rodrigues AD (1999) Integrated cytochrome P450 reaction phenotyping: attempting to bridge the gap between cDNA-expressed cytochromes P450 and native human liver microsomes. Biochem Pharmacol 57(5):465–480. https://doi.org/10.1016/s0006-2952(98)00268-8
Rüdesheim S, Wojtyniak JG, Selzer D, Hanke N, Mahfoud F, Schwab M, Lehr T (2020) Physiologically based pharmacokinetic modeling of metoprolol enantiomers and α-hydroxymetoprolol to describe CYP2D6 drug-gene interactions. Pharmaceutics 12(12):1200. https://doi.org/10.3390/pharmaceutics12121200
Rüdesheim S, Selzer D, Fuhr U, Schwab M, Lehr T (2022) Physiologically-based pharmacokinetic modeling of dextromethorphan to investigate interindividual variability within CYP2D6 activity score groups. CPT Pharmacomet Syst Pharmacol 11(4):494–511. https://doi.org/10.1002/psp4.12776
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N (2015) Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos 43(11):1823–1837. https://doi.org/10.1124/dmd.115.065920
Shin HB, Jung EH, Kang P, Lim CW, Oh KY, Cho CK, Lee YJ, Choi CI, Jang CG, Lee SY, Bae JW (2020) ABCB1 c.2677G>T/c.3435C>T diplotype increases the early-phase oral absorption of losartan. Arch Pharm Res 43(11):1187–1196. https://doi.org/10.1007/s12272-020-01294-3
Song G, Chung JE, Yee J, Lee KE, Park K, Gwak HS (2021) Effects of SLCO1B1 and SLCO1B3 genetic polymorphisms on valsartan pharmacokinetics in healthy Korean volunteers. J Pers Med 11(9):862. https://doi.org/10.3390/jpm11090862
Stingl JC, Bartels H, Viviani R, Lehmann M, Brockmöller J (2014) Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: a quantitative systematic review. Pharmacol Ther 141(1):92–116. https://doi.org/10.1016/j.pharmthera.2013.09.002
Suwannakul S, Ieiri I, Kimura M, Kawabata K, Kusuhara H, Hirota T, Irie S, Sugiyama Y, Higuchi S (2008) Pharmacokinetic interaction between pravastatin and olmesartan in relation to SLCO1B1 polymorphism. J Hum Genet 53(10):899–904. https://doi.org/10.1007/s10038-008-0324-9
Tang C, Shou M, Rushmore TH, Mei Q, Sandhu P, Woolf EJ, Rose MJ, Gelmann A, Greenberg HE, De Lepeleire I, Hecken AV, De Schepper PJ, Ebel DL, Schwartz JI, Rodrigues AD (2001) In-vitro metabolism of celecoxib, a cyclooxygenase-2 inhibitor, by allelic variant forms of human liver microsomal cytochrome P450 2C9: correlation with CYP2C9 genotype and in-vivo pharmacokinetics. Pharmacogenetics 11(3):223–235. https://doi.org/10.1097/00008571-200104000-00006
Tanveer A, Hussain K, Tasneem H, Arif I, Rashid M, Abbas N, Shamim R, Shah PA, Bukhari NI (2022) Prediction of CYP-mediated silybin A-losartan pharmacokinetic interactions using physiological based pharmacokinetic modeling. J Pharmacokinet Pharmacodyn 49(3):311–323. https://doi.org/10.1007/s10928-022-09804-0
Wang L, Bao SH, Pan PP, Xia MM, Chen MC, Liang BQ, Dai DP, Cai JP, Hu GX (2015) Effect of CYP2C9 genetic polymorphism on the metabolism of flurbiprofen in vitro. Drug Dev Ind Pharm 41(8):1363–1367. https://doi.org/10.3109/03639045.2014.950274
Weinshilboum R (2003) Inheritance and drug response. N Engl J Med 348(6):529–537. https://doi.org/10.1056/NEJMra020021
Wen SY, Wang H, Sun OJ, Wang SQ (2003) Rapid detection of the known SNPs of CYP2C9 using oligonucleotide microarray. World J Gastroenterol: World J Gastroenterol 9(6):1342–1346. https://doi.org/10.3748/wjg.v9.i6.1342
Whang SS, Cho CK, Jung EH, Kang P, Park HJ, Lee YJ, Choi CI, Bae JW, Kim HS, Jang CG, Lee SY (2022) Physiologically based pharmacokinetic (PBPK) modeling of flurbiprofen in different CYP2C9 genotypes. Arch Pharm Res 45(8):584–595. https://doi.org/10.1007/s12272-022-01403-4
World Health Organization (2021) The global health observatory: indicator metadata registry list: blood pressure/hypertension. https://www.who.int/data/gho/indicator-metadata-registry/imr-details/3155. Accessed 06 Nov 2023
Yang H, Yang L, Zhong X, Jiang X, Zheng L, Wang L (2022) Physiologically based pharmacokinetic modeling of brivaracetam and its interactions with rifampin based on CYP2C19 phenotypes. Eur J Pharm Sci 177:106258. https://doi.org/10.1016/j.ejps.2022.106258
Zhuang X, Lu C (2016) PBPK modeling and simulation in drug research and development. Acta Pharm Sin B 6(5):430–440. https://doi.org/10.1016/j.apsb.2016.04.004
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (Grant No. NRF-2019R1A2C1004582).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cho, CK., Kang, P., Jang, CG. et al. Physiologically based pharmacokinetic (PBPK) modeling to predict the pharmacokinetics of irbesartan in different CYP2C9 genotypes. Arch. Pharm. Res. 46, 939–953 (2023). https://doi.org/10.1007/s12272-023-01472-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-023-01472-z