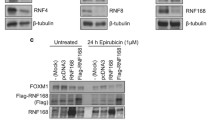
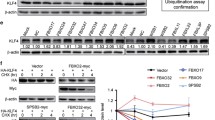
Abstract
E2F 1, 2, and 3a, (refer to as E2Fs) are a subfamily of E2F transcription factor family that play essential roles in cell-cycle progression, DNA replication, DNA repair, apoptosis, and differentiation. Although the transcriptional regulation of E2Fs has focused on pocket protein retinoblastoma protein complex, recent studies indicate that post-translational modification and stability regulation of E2Fs play key roles in diverse cellular processes. In this study, we found that FBXO1, a component of S-phase kinase-associated protein 1 (SKP1)-cullin 1-F-box protein (SCF) complex, is an E2Fs binding partner. Furthermore, FBXO1 to E2Fs binding induced K48 ubiquitination and subsequent proteasomal degradation of E2Fs. Binding domain analysis indicated that the Arg (R)/Ile (I) and R/Val (V) motifs, which are located in the dimerization domain of E2Fs, of E2F 1 and 3a and E2F2, respectively, acted as degron motifs (DMs) for FBXO1. Notably, RI/AA or RV/AA mutation in the DMs reduced FBXO1-mediated ubiquitination and prolonged the half-lives of E2Fs. Importantly, the stabilities of E2Fs were affected by phosphorylation of threonine residues located near RI and RV residues of DMs. Phosphorylation prediction database analysis and specific inhibitor analysis revealed that MEK/ERK signaling molecules play key roles in FBXO1/E2Fs’ interaction and modulate E2F protein turnover. Moreover, both elevated E2Fs protein levels by knockdown of FBXO1 and decreased E2Fs protein levels by sh-E2F3a delayed G1/S cell cycle transition, resulting in inhibition of cancer cell proliferation. These results demonstrated that FBXO1-E2Fs axis-mediated precise E2Fs stability regulation plays a key role in cell proliferation via G1/S cell cycle transition.





Similar content being viewed by others
References
Akiyama T, Ohuchi T, Sumida S, Matsumoto K, Toyoshima K (1992) Phosphorylation of the retinoblastoma protein by cdk2. Proc Natl Acad Sci USA 89:7900–7904. https://doi.org/10.1073/pnas.89.17.7900
An HJ, Lee CJ, Lee GE, Choi Y, Jeung D, Chen W, Lee HS, Kang HC, Lee JY, Kim DJ, Choi JS, Cho ES, Choi JS, Cho YY (2022) FBXW7-mediated ERK3 degradation regulates the proliferation of lung cancer cells. Exp Mol Med 54:35–46. https://doi.org/10.1038/s12276-021-00721-9
Augustine T, Chaudhary P, Gupta K, Islam S, Ghosh P, Santra MK, Mitra D (2017) Cyclin F/FBXO1 interacts with HIV-1 viral infectivity factor (vif) and restricts progeny virion infectivity by ubiquitination and proteasomal degradation of vif protein through SCF(cyclin F) E3 ligase machinery. J Biol Chem 292:5349–5363. https://doi.org/10.1074/jbc.M116.765842
Bagchi S, Weinmann R, Raychaudhuri P (1991) The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell 65:1063–1072. https://doi.org/10.1016/0092-8674(91)90558-g
Bai C, Richman R, Elledge SJ (1994) Human cyclin F. EMBO J 13:6087–6098
Berkovich E, Ginsberg D (2003) ATM is a target for positive regulation by E2F-1. Oncogene 22:161–167. https://doi.org/10.1038/sj.onc.1206144
Bertoli C, Skotheim JM, De Bruin RA (2013) Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 14:518–528. https://doi.org/10.1038/nrm3629
Boekhout M, Yuan R, Wondergem AP, Segeren HA, Van Liere EA, Awol N, Jansen I, Wolthuis RM, De Bruin A, Westendorp B (2016) Feedback regulation between atypical E2Fs and APC/CCdh1 coordinates cell cycle progression. EMBO Rep 17:414–427. https://doi.org/10.15252/embr.201540984
Cheng F, De Luca A, Hogan AL, Rayner SL, Davidson JM, Watchon M, Stevens CH, Munoz SS, Ooi L, Yerbury JJ, Don EK, Fifita JA, Villalva MD, Suddull H, Chapman TR, Hedl TJ, Walker AK, Yang S, Morsch M, Shi B, Blair IP, Laird AS, Chung RS, Lee A (2021) Unbiased label-free quantitative proteomics of cells expressing amyotrophic lateral sclerosis (ALS) mutations in CCNF reveals activation of the apoptosis pathway: a workflow to screen pathogenic gene mutations. Front Mol Neurosci 14:627740. https://doi.org/10.3389/fnmol.2021.627740
Chia R, Chio A, Traynor BJ (2018) Novel genes associated with amyotrophic lateral sclerosis: diagnostic and clinical implications. Lancet Neurol 17:94–102. https://doi.org/10.1016/S1474-4422(17)30401-5
Choudhury R, Bonacci T, Wang X, Truong A, Arceci A, Zhang Y, Mills CA, Kernan JL, Liu P, Emanuele MJ (2017) The E3 ubiquitin ligase SCF(cyclin F) transmits AKT signaling to the cell-cycle machinery. Cell Rep 20:3212–3222. https://doi.org/10.1016/j.celrep.2017.08.099
Clijsters L, Hoencamp C, Calis JJA, Marzio A, Handgraaf SM, Cuitino MC, Rosenberg BR, Leone G, Pagano M (2019) Cyclin F controls cell-cycle transcriptional outputs by directing the degradation of the three activator E2Fs. Mol Cell 74:1264-1277e7. https://doi.org/10.1016/j.molcel.2019.04.010
Connell-Crowley L, Harper JW, Goodrich DW (1997) Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell 8:287–301. https://doi.org/10.1091/mbc.8.2.287
D’angiolella V, Donato V, Vijayakumar S, Saraf A, Florens L, Washburn MP, Dynlacht B, Pagano M (2010) SCF(cyclin F) controls centrosome homeostasis and mitotic fidelity through CP110 degradation. Nature 466:138–142. https://doi.org/10.1038/nature09140
D’angiolella V, Donato V, Forrester FM, Jeong YT, Pellacani C, Kudo Y, Saraf A, Florens L, Washburn MP, Pagano M (2012) Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell 149:1023–1034. https://doi.org/10.1016/j.cell.2012.03.043
D’angiolella V, Esencay M, Pagano M (2013) A cyclin without cyclin-dependent kinases: cyclin F controls genome stability through ubiquitin-mediated proteolysis. Trends Cell Biol 23:135–140. https://doi.org/10.1016/j.tcb.2012.10.011
Dankert JF, Rona G, Clijsters L, Geter P, Skaar JR, Bermudez-Hernandez K, Sassani E, Fenyo D, Ueberheide B, Schneider R, Pagano M (2016) Cyclin F-Mediated degradation of SLBP limits H2A.X accumulation and apoptosis upon genotoxic stress in G2. Mol Cell 64:507–519. https://doi.org/10.1016/j.molcel.2016.09.010
Elia AE, Boardman AP, Wang DC, Huttlin EL, Everley RA, Dephoure N, Zhou C, Koren I, Gygi SP, Elledge SJ (2015) Quantitative proteomic atlas of ubiquitination and acetylation in the DNA damage response. Mol Cell 59:867–881. https://doi.org/10.1016/j.molcel.2015.05.006
Emanuele MJ, Elia AE, Xu Q, Thoma CR, Izhar L, Leng Y, Guo A, Chen YN, Rush J, Hsu PW, Yen HC, Elledge SJ (2011) Global identification of modular cullin-RING ligase substrates. Cell 147:459–474. https://doi.org/10.1016/j.cell.2011.09.019
Enrico TP, Stallaert W, Wick ET, Ngoi P, Wang X, Rubin SM, Brown NG, Purvis JE, Emanuele MJ (2021) Cyclin F drives proliferation through SCF-dependent degradation of the retinoblastoma-like tumor suppressor p130/RBL2. Elife. https://doi.org/10.7554/eLife.70691
Hiebert SW, Chellappan SP, Horowitz JM, Nevins JR (1992) The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev 6:177–185. https://doi.org/10.1101/gad.6.2.177
Hofmann F, Martelli F, Livingston DM, Wang Z (1996) The retinoblastoma gene product protects E2F-1 from degradation by the ubiquitin-proteasome pathway. Genes Dev 10:2949–2959. https://doi.org/10.1101/gad.10.23.2949
Ivanova IA, Nakrieko KA, Dagnino L (2009) Phosphorylation by p38 MAP kinase is required for E2F1 degradation and keratinocyte differentiation. Oncogene 28:52–62. https://doi.org/10.1038/onc.2008.354
Kitagawa M, Higashi H, Suzuki-Takahashi I, Segawa K, Hanks SK, Taya Y, Nishimura S, Okuyama A (1995) Phosphorylation of E2F–1 by cyclin A-cdk2. Oncogene 10:229–36
Klein DK, Hoffmann S, Ahlskog JK, O’hanlon K, Quaas M, Larsen BD, Rolland B, Rosner HI, Walter D, Kousholt AN, Menzel T, Lees M, Johansen JV, Rappsilber J, Engeland K, Sorensen CS (2015) Cyclin F suppresses B-Myb activity to promote cell cycle checkpoint control. Nat Commun 6:5800. https://doi.org/10.1038/ncomms6800
Lan H, Tang Z, Jin H, Sun Y (2016) Neddylation inhibitor MLN4924 suppresses growth and migration of human gastric cancer cells. Sci Rep 6:24218. https://doi.org/10.1038/srep24218
Lee A, Rayner SL, Gwee SSL, De Luca A, Shahheydari H, Sundaramoorthy V, Ragagnin A, Morsch M, Radford R, Galper J, Freckleton S, Shi B, Walker AK, Don EK, Cole NJ, Yang S, Williams KL, Yerbury JJ, Blair IP, Atkin JD, Molloy MP, Chung RS (2018) Pathogenic mutation in the ALS/FTD gene, CCNF, causes elevated Lys48-linked ubiquitylation and defective autophagy. Cell Mol Life Sci 75:335–354. https://doi.org/10.1007/s00018-017-2632-8
Lee CJ, An HJ, Kim SM, Yoo SM, Park J, Lee GE, Kim WY, Kim DJ, Kang HC, Lee JY, Lee HS, Cho SJ, Cho YY (2020) FBXW7-mediated stability regulation of signal transducer and activator of transcription 2 in melanoma formation. Proc Natl Acad Sci USA 117:584–594. https://doi.org/10.1073/pnas.1909879116
Lee GE, Lee CJ, An HJ, Kang HC, Lee HS, Lee JY, Oh SR, Cho SJ, Kim DJ, Cho YY (2021) Fargesin inhibits EGF-induced cell transformation and colon cancer cell growth by suppression of CDK2/Cyclin E signaling pathway. Int J Mol Sci. https://doi.org/10.3390/ijms22042073
Liu Y, Pan B, Qu W, Cao Y, Li J, Zhao H (2021) Systematic analysis of the expression and prognosis relevance of FBXO family reveals the significance of FBXO1 in human breast cancer. Cancer Cell Int 21:130. https://doi.org/10.1186/s12935-021-01833-y
Lv Y, Xiao J, Liu J, Xing F (2017) E2F8 is a potential therapeutic target for hepatocellular carcinoma. J Cancer 8:1205–1213. https://doi.org/10.7150/jca.18255
Martelli F, Livingston DM (1999) Regulation of endogenous E2F1 stability by the retinoblastoma family proteins. Proc Natl Acad Sci USA 96:2858–2863. https://doi.org/10.1073/pnas.96.6.2858
Pan C, Jiao B, Xiao T, Hou L, Zhang W, Liu X, Xu J, Tang B, Shen L (2017) Mutations of CCNF gene is rare in patients with amyotrophic lateral sclerosis and frontotemporal dementia from Mainland China. Amyotroph Lateral Scler Frontotemporal Degener 18:265–268. https://doi.org/10.1080/21678421.2017.1293111
Pasinelli P, Brown RH (2006) Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci 7:710–723. https://doi.org/10.1038/nrn1971
Ren B, Cam H, Takahashi Y, Volkert T, Terragni J, Young RA, Dynlacht BD (2002) E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev 16:245–256. https://doi.org/10.1101/gad.949802
Sekine M, Ito M, Uemukai K, Maeda Y, Nakagami H, Shinmyo A (1999) Isolation and characterization of the E2F-like gene in plants. FEBS Lett 460:117–122. https://doi.org/10.1016/s0014-5793(99)01296-x
Sharma SS, Ma L, Bagui TK, Forinash KD, Pledger WJ (2012) A p27Kip1 mutant that does not inhibit CDK activity promotes centrosome amplification and micronucleation. Oncogene 31:3989–3998. https://doi.org/10.1038/onc.2011.550
Takahashi Y, Rayman JB, Dynlacht BD (2000) Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev 14:804–16
Tripolszki K, Gampawar P, Schmidt H, Nagy ZF, Nagy D, Klivenyi P, Engelhardt JI, Szell M (2019) Comprehensive genetic analysis of a hungarian amyotrophic lateral sclerosis cohort. Front Genet 10:732. https://doi.org/10.3389/fgene.2019.00732
Van Den Heuvel S, Dyson NJ (2008) Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 9:713–724. https://doi.org/10.1038/nrm2469
Walter D, Hoffmann S, Komseli ES, Rappsilber J, Gorgoulis V, Sorensen CS (2016) SCF(cyclin F)-dependent degradation of CDC6 suppresses DNA re-replication. Nat Commun 7:10530. https://doi.org/10.1038/ncomms10530
Xu M, Sheppard KA, Peng CY, Yee AS, Piwnica-Worms H (1994) Cyclin A/CDK2 binds directly to E2F-1 and inhibits the DNA-binding activity of E2F-1/DP-1 by phosphorylation. Mol Cell Biol 14:8420–8431. https://doi.org/10.1128/mcb.14.12.8420-8431.1994
Xue Y, Ren J, Gao X, Jin C, Wen L, Yao X (2008) GPS 2.0, a tool to predict kinase-specific phosphorylation sites in hierarchy. Mol Cell Proteomics 7:1598–1608. https://doi.org/10.1074/mcp.M700574-MCP200
Zhao J, Ramos R, Demma M (2013) CDK8 regulates E2F1 transcriptional activity through S375 phosphorylation. Oncogene 32:3520–3530. https://doi.org/10.1038/onc.2012.364
Acknowledgements
This research was funded by the Research Fund of The Catholic University of Korea (Grant no. M-2020‐B00014‐00082), the Ministry of Science, ICT, and Future Planning (Grant nos. NRF-2020R1A2B5B02001804 and NRF-2020R1A4A2002894).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, GE., Jeung, D., Chen, W. et al. MEKs/ERKs-mediated FBXO1/E2Fs interaction interference modulates G1/S cell cycle transition and cancer cell proliferation. Arch. Pharm. Res. 46, 44–58 (2023). https://doi.org/10.1007/s12272-023-01426-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-023-01426-5