Abstract
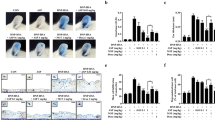
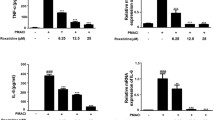
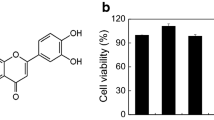
Mast cells play essential role in allergic reactions through the process called mast cell degranulation. Recent studies have found that a basic secretagogue compound 48/80 (C48/80) induces non-IgE-mediated mast cell degranulation via activation of human Mas-related G protein-coupled receptor X2 (MRGPRX2) and mouse MrgprB2. Although previous studies have revealed that caffeic acid (CA) and its derivatives possess anti-allergic effects via IgE-dependent manner, it is largely elusive whether these compounds have impact on MRGPRX2/MrgprB2 to exert inhibitory effects. Therefore, the present study investigated whether CA as well as its derivatives – rosmarinic acid (RA) and caffeic acid phenethyl ester (CAPE) – has the ability to inhibit the activity of MRGPRX2/MrgprB2 to evoke pseudo-allergic effects. As a result, it was found that CAPE inhibits C48/80-induced activation of MRGPRX2/MrgprB2, but neither CA nor RA showed discernible inhibition. Furthermore, the β-hexosaminidase release assay showed that CAPE inhibits mouse peritoneal mast cell degranulation in both IgE-dependent and MrgprB2-dependent manners. Additionally, mouse paw edema induced by C48/80 was dramatically suppressed by co-treatment of CAPE, suggesting that CAPE possesses a protective effect on C48/80-evoked pseudo-allergic reactions. The pretreatment of CAPE also significantly decreased scratching bouts of mice evoked by C48/80, demonstrating that CAPE also has an anti-pruritic effect. Therefore, these data implicate that CAPE can suppress pseudo-allergic reactions evoked by C48/80 via MrgprB2-dependent manner. Finally, molecular docking analysis showed that CAPE is predicted to bind to human MRGPRX2 in the region where C48/80 also binds, implying that CAPE can be a competitive inhibitor of MRGPRX2. In conclusion, it is found that CAPE has the ability to inhibit MRGPRX2/MrgprB2, leading to the prevention of mast cell degranulation and further to the alleviation of mast cell reactions. These results indicate that CAPE as a CA derivative could be developed as a new protective agent that exerts dual inhibition of mast cell degranulation mediated by IgE and MRGPRX2/MrgprB2.







Similar content being viewed by others
References
Ali H (2017) Emerging roles for MAS-Related G protein-coupled receptor-X2 in host defense peptide, opioid, and neuropeptide-mediated inflammatory reactions. Adv Immunol 136:123–162. https://doi.org/10.1016/bs.ai.2017.06.002
Azimi E, Reddy VB, Shade KC, Anthony RM, Talbot S, Pereira PJS, Lerner EA (2016) Dual action of neurokinin-1 antagonists on Mas-related GPCRs. JCI Insight 1:e89362. https://doi.org/10.1172/jci.insight.89362
Callahan BN, Kammala AK, Syed M, Yang C, Occhiuto CJ, Nellutla R, Chumanevich AP, Oskeritzian CA, Das R, Subramanian H (2020) Osthole, a natural plant derivative inhibits MRGPRX2 induced mast cell responses. Front Immunol 11:703. https://doi.org/10.3389/fimmu.2020.00703
Chajra H, Nadim M, Auriol D, Schweikert K, Lefevre F (2015) Combination of new multifunctional molecules for erythematotelangiectatic rosacea disorder. Clin Cosmet Investig Dermatol 8:501–510. https://doi.org/10.2147/CCID.S92326
Cho MS, Park WS, Jung WK, Qian ZJ, Lee DS, Choi JS, Lee DY, Park SG, Seo SK, Kim HJ, Won JY, Yu BC, Choi IW (2014) Caffeic acid phenethyl ester promotes anti-inflammatory effects by inhibiting MAPK and NF-kappaB signaling in activated HMC-1 human mast cells. Pharm Biol 52:926–932. https://doi.org/10.3109/13880209.2013.865243
Da Cunha FM, Duma D, Assreuy J, Buzzi FC, Niero R, Campos MM, Calixto JB (2004) Caffeic acid derivatives: in vitro and in vivo anti-inflammatory properties. Free Radic Res 38:1241–1253. https://doi.org/10.1080/10715760400016139
Demestre M, Messerli SM, Celli N, Shahhossini M, Kluwe L, Mautner V, Maruta H (2009) CAPE (caffeic acid phenethyl ester)-based propolis extract (Bio 30) suppresses the growth of human neurofibromatosis (NF) tumor xenografts in mice. Phytother Res 23:226–230. https://doi.org/10.1002/ptr.2594
Fan JY, Fu AL, Zhang L (2019) Progress in molecular docking. Quant Biol 7:83–89. https://doi.org/10.1007/s40484-019-0172-y
Fesen MR, Kohn KW, Leteurtre F, Pommier Y (1993) Inhibitors of human immunodeficiency virus integrase. Proc Natl Acad Sci USA 90:2399–2403. https://doi.org/10.1073/pnas.90.6.2399
Grosdidier A, Zoete V, Michielin O (2011) SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res 39,:W270–W277. https://doi.org/10.1093/nar/gkr366
Hashimoto H, Messerli SM, Sudo T, Maruta H (2009) Ivermectin inactivates the kinase PAK1 and blocks the PAK1-dependent growth of human ovarian cancer and NF2 tumor cell lines. Drug Discov Ther 3:243–246
Hosnuter M, Gurel A, Babuccu O, Armutcu F, Kargi E, Isikdemir A (2004) The effect of CAPE on lipid peroxidation and nitric oxide levels in the plasma of rats following thermal injury. Burns 30:121–125. https://doi.org/10.1016/j.burns.2003.09.022
Hou Y, Che D, Ma P, Zhao T, Zeng Y, Wang N (2018) Anti-pseudo-allergy effect of isoliquiritigenin is MRGPRX2-dependent. Immunol Lett 198:52–59. https://doi.org/10.1016/j.imlet.2018.04.004
Jeong JY, Yim HS, Ryu JY, Lee HS, Lee JH, Seen DS, Kang SG (2012) One-step sequence- and ligation-independent cloning as a rapid and versatile cloning method for functional genomics studies. Appl Environ Microbiol 78:5440–5443. https://doi.org/10.1128/AEM.00844-12
Jeong H, Shin JY, Lee K, Lee SJ, Chong HJ, Jeong H, Jeon YE, Shin DS, Jang S, Kim KH, Kim SI, Lee YS, Ju BG (2020) Caffeoyl-prolyl-histidine amide inhibits Fyn and alleviates atopic dermatitis-like phenotypes via suppression of NF-kappaB activation. Int J Mol Sci. https://doi.org/10.3390/ijms21197160
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, Bates R, Zidek A, Potapenko A, Bridgland A, Meyer C, Kohl SaA, Ballard AJ, Cowie A, Romera-Paredes B, Nikolov S, Jain R, Adler J, Back T, Petersen S, Reiman D, Clancy E, Zielinski M, Steinegger M, Pacholska M, Berghammer T, Bodenstein S, Silver D, Vinyals O, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D (2021) Highly accurate protein structure prediction with AlphaFold. Nature 596:583–589. https://doi.org/10.1038/s41586-021-03819-2
Jung WK, Lee DY, Choi YH, Yea SS, Choi I, Park SG, Seo SK, Lee SW, Lee CM, Kim SK, Jeon YJ, Choi IW (2008) Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma. Life Sci 82:797–805. https://doi.org/10.1016/j.lfs.2008.01.014
Kang NJ, Lee KW, Shin BJ, Jung SK, Hwang MK, Bode AM, Heo YS, Lee HJ, Dong Z (2009) Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 30:321–330. https://doi.org/10.1093/carcin/bgn282
Lee HS, Lee SY, Park SH, Lee JH, Ahn SK, Choi YM, Choi DJ, Chang JH (2013) Antimicrobial medical sutures with caffeic acid phenethyl ester and their in vitro/in vivo biological assessment. Medchemcomm 4:777–782. https://doi.org/10.1039/c2md20289a
Liao HF, Chen YY, Liu JJ, Hsu ML, Shieh HJ, Liao HJ, Shieh CJ, Shiao MS, Chen YJ (2003) Inhibitory effect of caffeic acid phenethyl ester on angiogenesis, tumor invasion, and metastasis. J Agric Food Chem 51:7907–7912. https://doi.org/10.1021/jf034729d
Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X (2009) Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139:1353–1365. https://doi.org/10.1016/j.cell.2009.11.034
Mcneil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X (2015) Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519:237–241. https://doi.org/10.1038/nature14022
Mcneil BD (2021) MRGPRX2 and adverse drug reactions. Front Immunol 12:676354. https://doi.org/10.3389/fimmu.2021.676354
Meixiong J, Anderson M, Limjunyawong N, Sabbagh MF, Hu E, Mack MR, Oetjen LK, Wang F, Kim BS, Dong X (2019) Activation of mast-cell-expressed mas-related G-protein-coupled receptors drives non-histaminergic itch. Immunity 50:1163–1171e5. https://doi.org/10.1016/j.immuni.2019.03.013
Nader MA (2013) Caffeic acid phenethyl ester attenuates IgE-induced immediate allergic reaction. Inflammopharmacology 21:169–176. https://doi.org/10.1007/s10787-012-0138-4
Natarajan K, Singh S, Burke TR Jr, Grunberger D, Aggarwal BB (1996) Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA 93:9090–9095. https://doi.org/10.1073/pnas.93.17.9090
Navines-Ferrer A, Ainsua-Enrich E, Serrano-Candelas E, Proano-Perez E, Munoz-Cano R, Gastaminza G, Olivera A, Martin M (2021) MYO1F regulates IgE and MRGPRX2-dependent mast cell exocytosis. J Immunol 206:2277–2289. https://doi.org/10.4049/jimmunol.2001211
Ogasawara H, Furuno M, Edamura K, Noguchi M (2019) Novel MRGPRX2 antagonists inhibit IgE-independent activation of human umbilical cord blood-derived mast cells. J Leukoc Biol 106:1069–1077. https://doi.org/10.1002/JLB.2AB1018-405R
Ozturk G, Ginis Z, Akyol S, Erden G, Gurel A, Akyol O (2012) The anticancer mechanism of caffeic acid phenethyl ester (CAPE): review of melanomas, lung and prostate cancers. Eur Rev Med Pharmacol Sci 16:2064–2068
Park SG, Lee DY, Seo SK, Lee SW, Kim SK, Jung WK, Kang MS, Choi YH, Yea SS, Choi I, Choi IW (2008) Evaluation of anti-allergic properties of caffeic acid phenethyl ester in a murine model of systemic anaphylaxis. Toxicol Appl Pharmacol 226:22–29. https://doi.org/10.1016/j.taap.2007.08.003
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. https://doi.org/10.1002/jcc.20084
Pradhananga S, Shim WS (2015) Caffeic acid exhibits anti-pruritic effects by inhibition of multiple itch transmission pathways in mice. Eur J Pharmacol 762:313–321. https://doi.org/10.1016/j.ejphar.2015.06.006
Pullen NA, Falanga YT, Morales JK, Ryan JJ (2012) The Fyn-STAT5 pathway: a new frontier in IgE- and IgG-mediated mast cell signaling. Front Immunol 3:117. https://doi.org/10.3389/fimmu.2012.00117
Redegeld FA, Yu Y, Kumari S, Charles N, Blank U (2018) Non-IgE mediated mast cell activation. Immunol Rev 282:87–113. https://doi.org/10.1111/imr.12629
Shinohara T, Harada M, Ogi K, Maruyama M, Fujii R, Tanaka H, Fukusumi S, Komatsu H, Hosoya M, Noguchi Y, Watanabe T, Moriya T, Itoh Y, Hinuma S (2004) Identification of a G protein-coupled receptor specifically responsive to beta-alanine. J Biol Chem 279:23559–23564. https://doi.org/10.1074/jbc.M314240200
Tsvilovskyy V, Solis-Lopez A, Ohlenschlager K, Freichel M (2018) Isolation of peritoneum-derived mast cells and their functional characterization with Ca2+-imaging and degranulation assays. J Vis Exp. https://doi.org/10.3791/57222
Varadi M, Anyango S, Deshpande M, Nair S, Natassia C, Yordanova G, Yuan D, Stroe O, Wood G, Laydon A, Zidek A, Green T, Tunyasuvunakool K, Petersen S, Jumper J, Clancy E, Green R, Vora A, Lutfi M, Figurnov M, Cowie A, Hobbs N, Kohli P, Kleywegt G, Birney E, Hassabis D, Velankar S (2022) AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res 50:D439–D444. https://doi.org/10.1093/nar/gkab1061
Wagh VD (2013) Propolis: a wonder bees product and its pharmacological potentials. Adv Pharmacol Sci, 2013, 308249. https://doi.org/10.1155/2013/308249
Wang N, Che D, Zhang T, Liu R, Cao J, Wang J, Zhao T, Ma P, Dong X, He L (2018) Saikosaponin A inhibits compound 48/80-induced pseudo-allergy via the Mrgprx2 pathway in vitro and in vivo. Biochem Pharmacol 148:147–154. https://doi.org/10.1016/j.bcp.2017.12.017
Wang J, Zhang Y, Li C, Ding Y, Hu S, An H (2020a) Inhibitory function of Shikonin on MRGPRX2-mediated pseudo-allergic reactions induced by the secretagogue. Phytomedicine 68:153149. https://doi.org/10.1016/j.phymed.2019.153149
Wang J, Zhang Y, Wang J, Liu R, Zhang G, Dong K, Zhang T (2020b) Paeoniflorin inhibits MRGPRX2-mediated pseudo-allergic reaction via calcium signaling pathway. Phytother Res 34:401–408. https://doi.org/10.1002/ptr.6531
Wang J, Zhang Y, Hu S, Ge S, Jia M, Wang N (2021a) Resveratrol inhibits MRGPRX2-mediated mast cell activation via Nrf2 pathway. Int Immunopharmacol 93:107426. https://doi.org/10.1016/j.intimp.2021.107426
Wang N, Wang J, Zhang Y, Zeng Y, Hu S, Bai H, Hou Y, Wang C, He H, He L (2021b) Imperatorin ameliorates mast cell-mediated allergic airway inflammation by inhibiting MRGPRX2 and CamKII/ERK signaling pathway. Biochem Pharmacol 184:114401. https://doi.org/10.1016/j.bcp.2020.114401
Weiler CR (2019) Mastocytosis, quinolones, MRGPRX2, and anaphylaxis. J Allergy Clin Immunol Pract 7:2091–2092. https://doi.org/10.1016/j.jaip.2019.02.015
Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM (2011) TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 14:595–602. https://doi.org/10.1038/nn.2789
Wolf K, Kuhn H, Boehm F, Gebhardt L, Glaudo M, Agelopoulos K, Stander S, Ectors P, Zahn D, Riedel YK, Thimm D, Muller CE, Kretschmann S, Kremer AN, Chien D, Limjunyawong N, Peng Q, Dong X, Kolkhir P, Scheffel J, Sogaard ML, Weigmann B, Neurath MF, Hawro T, Metz M, Fischer MJM, Kremer AE (2021) A group of cationic amphiphilic drugs activates MRGPRX2 and induces scratching behavior in mice. J Allergy Clin Immunol 148:506-522. https://doi.org/10.1016/j.jaci.2020.12.655
Yang F, Guo L, Li Y, Wang G, Wang J, Zhang C, Fang GX, Chen X, Liu L, Yan X, Liu Q, Qu C, Xu Y, Xiao P, Zhu Z, Li Z, Zhou J, Yu X, Gao N, Sun JP (2021) Structure, function and pharmacology of human itch receptor complexes. Nature 600:164–169. https://doi.org/10.1038/s41586-021-04077-y
Zhang Y, Wang J, Ge S, Zeng Y, Wang N, Wu Y (2020) Roxithromycin inhibits compound 48/80-induced pseudo-allergy via the MrgprX2 pathway both in vitro and in vivo. Cell Immunol 358:104239. https://doi.org/10.1016/j.cellimm.2020.104239
Zhu F, Xu Z, Yonekura L, Yang R, Tamura H (2015) Antiallergic activity of rosmarinic acid esters is modulated by hydrophobicity, and bulkiness of alkyl side chain. Biosci Biotechnol Biochem 79:1178–1182. https://doi.org/10.1080/09168451.2015.1010478
Acknowledgements
This study was supported by the Gachon University research fund of 202008420009, and a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (2021R1A2C1005865).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adhikari, N., Shim, WS. Caffeic acid phenethyl ester inhibits pseudo-allergic reactions via inhibition of MRGPRX2/MrgprB2-dependent mast cell degranulation. Arch. Pharm. Res. 45, 644–657 (2022). https://doi.org/10.1007/s12272-022-01405-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-022-01405-2