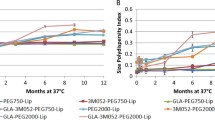
Abstract
Adjuvants are essential vaccine components used to enhance, accelerate, and/or prolong adaptive immunity against specific vaccine antigens. In this study, we compared the adjuvanticity of two adjuvant formulations containing de-O-acylated lipooligosaccharide (dLOS), a toll-like receptor 4 agonist, on the Japanese encephalitis (JE) vaccine in mice. Mice were immunized once or twice at a two-week interval with inactivated JE vaccine in the absence or presence of adjuvant. We found that both the alum- and the liposome-based formulation induced significantly faster and higher serum IgG antibody responses as compared with the non-adjuvanted vaccine after either one or two immunizations. The antibody titers of the mouse immune sera correlated with 50% plaque reduction neutralization test (PRNT50) antibody titers. In addition, the dLOS/liposome formulation was more effective in inducing a Th1-type immune response than the dLOS/alum formulation, as suggested by a strong antigen-specific interferon (IFN)-γ response. Based on these results, we suggest that both alum- and liposome-based adjuvant formulations containing dLOS may be used for the development of JE vaccines with improved immunogenicity.






Similar content being viewed by others
References
Abe M, Kuzuhara S, Kino Y (2003) Establishment of an analyzing method for a Japanese encephalitis virus neutralization test in Vero cells. Vaccine 21:1989–1994
Barr TA, Brown S, Mastroeni P, Gray D (2009) B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol 183:1005–1012
Beasley DWC, Lewthwaite P, Solomon T (2008) Current use and development of vaccines for Japanese encephalitis. Expert Opin Biol Ther 8:95–106
Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, Ginsburg AS (2011) Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 89:766–774
Chen HW, Huang HW, Hu HM, Chung HH, Wu SH, Chong P, Tao MH, Pan CH (2014) A poorly neutralizing IgG2a/c response elicited by a DNA vaccine protects mice against Japanese encephalitis virus. J Gen Virol 95:1983–1990
Chen HL, Chang JK, Tang RB (2015) Current recommendations for the Japanese encephalitis vaccine. J Chin Med Assoc 78:271–275
Cho YJ, Ahn BY, Lee NG, Lee DH, Kim DS (2006) A combination of E. coli DNA fragments and modified lipopolysaccharides as a cancer immunotherapy. Vaccine 24:5862–5871
Di Pasquale A, Preiss S, Da Silva FT, Garcon N (2015) Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines 3:320–343
Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K (2009) Past, present, and future of Japanese encephalitis. Emerg Infect Dis 15:1–7
Halstead SB, Thomas SJ (2011) New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines 10:355–364
Han JE, Wui SR, Park SA, Lee NG, Kim KS, Cho YJ, Kim HJ, Kim HJ (2012) Comparison of the immune responses to the CIA06-adjuvanted human papillomavirus L1 VLP vaccine with those against the licensed HPV vaccine Cervarix™ in mice. Vaccine 30:4127–4134
Han JE, Wui SR, Kim KS, Cho YJ, Cho WJ, Lee NG (2014) Characterization of the structure and immunostimulatory activity of a vaccine adjuvant, de-O-acylated lipooligosaccharide. PLoS ONE 9:e85838
Kim SY, Lee SJ, Lim SJ (2014) Formulation and in vitro and in vivo evaluation of a cationic emulsion as a vehicle for improving adenoviral gene transfer. Int J Pharmaceut 475:49–59
Kimura-Kuroda J, Yasui K (1988) Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J Immunol 141:3606–3610
Korsholm KS, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, Geisler C, Andersen P (2007) The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology 121:216–226
Larena M, Regner M, Lee E, Lobigs M (2011) Pivotal role of antibody and subsidiary contribution of CD8+ T cells to recovery from infection in a murine model of Japanese encephalitis. J Virol 85:5446–5455
Larena M, Regner M, Lobigs M (2013) Cytolytic effector pathways and IFN-gamma help protect against Japanese encephalitis. Eur J Immunol 43:1789–1798
Lee CH, Tsai CM (1999) Quantification of bacterial lipopolysaccharides by the purpald assay: measuring formaldehyde generated from 2-keto-3-deoxyoctonate and heptose at the inner core by periodate oxidation. Anal Biochem 267:161–168
Lindblad EB (2004) Aluminium compounds for use in vaccines. Immunol Cell Biol 82:497–505
Lyons A, Kanesa-Thasan N, Kuschner RA, Eckels KH, Putnak R, Sun W, Burge R, Towle AC, Wilson P, Tauber E, Vaughn DW (2007) A Phase 2 study of a purified, inactivated virus vaccine to prevent Japanese encephalitis. Vaccine 25:3445–3453
Morefield GL, Jiang D, Romero-Mendez IZ, Geahlen RL, Hogenesch H, Hem SL (2005) Effect of phosphorylation of ovalbumin on adsorption by aluminum-containing adjuvants and elution upon exposure to interstitial fluid. Vaccine 23:1502–1506
Mount A, Koernig S, Silva A, Drane D, Maraskovsky E, Morelli AB (2013) Combination of adjuvants: the future of vaccine design. Expert Rev Vaccines 12:733–746
Romero-Steiner S, Fernandez J, Biltoft C, Wohl ME, Sanchez J, Feris J, Balter S, Levine OS, Carlone GM (2001) Functional antibody activity elicited by fractional doses of Haemophilus influenzae type b conjugate vaccine (polyribosylribitol phosphate-tetanus toxoid conjugate). Clin Diagn Lab Immun 8:1115–1119
Ryu JI, Park SA, Wui SR, Ko A, Han JE, Choi JA, Song MK, Kim KS, Cho YJ, Lee NG (2016) A de-O-acylated lipooligosaccharide-based adjuvant system promotes antibody and Th1-type immune responses to H1N1 pandemic influenza vaccine in mice. Biomed Res Int. https://doi.org/10.1155/2016/3713656
Ryu JI, Wui SR, Ko A, Lee YJ, Do H, Kim HJ, Rhee IM, Park SA, Kim KS, Cho YJ, Lee NG (2017) Increased immunogenicity and protective efficacy of a P. aeruginosa vaccine in mice using an alum and de-O-acylated lipooligosaccharide adjuvant system. J Microbiol Biotechnol 27:1539–1548
Skea DL, Barber BH (1993) Adhesion-mediated enhancement of the adjuvant activity of alum. Vaccine 11:1018–1026
Van Gessel Y, Klade CS, Putnak R, Formica A, Krasaesub S, Spruth M, Cena B, Tungtaeng A, Gettayacamin M, Dewasthaly S (2011) Correlation of protection against Japanese encephalitis virus and JE vaccine (IXIARO®) induced neutralizing antibody titers. Vaccine 29:5925–5931
Wiwanitkit V (2009) Development of a vaccine to prevent Japanese encephalitis: a brief review. Int J Gen Med 2:195–200
Wui SR, Kim HK, Han JE, Kim JM, Kim YH, Chun JH, Cho YJ, Lee NG (2011) A combination of the TLR4 agonist CIA05 and alum promotes the immune responses to Bacillus anthracis protective antigen in mice. Int Immunopharmacol 11:1195–1204
Wui SR, Han JE, Kim YH, Rhie GE, Lee NG (2013) Increased long-term immunity to Bacillus anthracis protective antigen in mice immunized with a CIA06B-adjuvanted anthrax vaccine. Arch Pharm Res 36:464–471
Acknowledgements
We thank Prof. J.H. Nam of Catholic University and Prof. B.L. Seong of Yonsei University (Republic of Korea) for providing the JEV Nakayama strain and recombinant JEV E protein, respectively. This study was supported by grants from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. HI14C2664 and No. HI13C0826).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
NG. Lee is an inventor of EyeGene-owned patents on dLOS-based adjuvants and a scientific advisor for EyeGene. The other authors have no conflicts of interests.
Rights and permissions
About this article
Cite this article
Ko, A., Wui, S.R., Ryu, J.I. et al. Comparison of the adjuvanticity of two adjuvant formulations containing de-O-acylated lipooligosaccharide on Japanese encephalitis vaccine in mice. Arch. Pharm. Res. 41, 219–228 (2018). https://doi.org/10.1007/s12272-017-0985-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0985-z