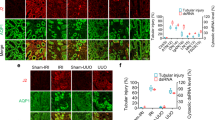
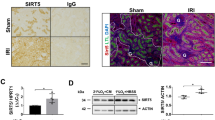
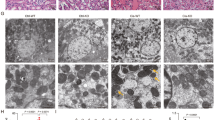
Abstract
Kidney ischemia and reperfusion injury (IRI) is associated with a high mortality rate, which is attributed to tubular oxidative stress and necrosis; however, an effective approach to limit IRI remains elusive. Spermidine, a naturally occurring polyamine, protects yeast cells against aging through the inhibition of oxidative stress and necrosis. In the present study, spermidine supplementation markedly attenuated increases in plasma creatinine concentration and tubular injury score after IRI. In addition, exogenous spermidine potently inhibited oxidative stress, especially lipid peroxidation after IRI in kidneys and exposure to hydrogen peroxide in kidney proximal tubular cells, suppressing plasma membrane disruption and necrosis. Consistent with spermidine supplementation, upregulation of ornithine decarboxylase (ODC) in human kidney proximal tubular cells significantly diminished lipid peroxidation and necrosis induced by hydrogen peroxide-induced injury. Conversely, ODC deficiency significantly enhanced lipid peroxidation and necrosis after exposure to hydrogen peroxide. Finally, small interfering RNA-mediated ODC inhibition induced functional and histological damage in kidneys as well as it increased lipid hydroperoxide levels after IRI. In conclusion, these data suggest that spermidine level determines kidney proximal tubular damage through oxidative stress and necrosis induced by IRI, and this finding provides a novel target for prevention of tubular damage induced by IRI.






Similar content being viewed by others
References
Adibhatla RM, Hatcher JF, Sailor K, Dempsey RJ (2002) Polyamines and central nervous system injury: spermine and spermidine decrease following transient focal cerebral ischemia in spontaneously hypertensive rats. Brain Res 938:81–86
Alhonen L, Rasanen TL, Sinervirta R, Parkkinen JJ, Korhonen VP, Pietila M, Janne J (2002) Polyamines are required for the initiation of rat liver regeneration. Biochem J 362:149–153
Ayala A, Munoz MF, Arguelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438
Barone S, Okaya T, Rudich S, Petrovic S, Tenrani K, Wang Z, Zahedi K, Casero RA, Lentsch AB, Soleimani M (2005) Distinct and sequential upregulation of genes regulating cell growth and cell cycle progression during hepatic ischemia-reperfusion injury. Am J Physiol Cell Physiol 289:C826–C835
Basile DP, Donohoe D, Roethe K, Osborn JL (2001) Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281:F887–F899
Baskaya MK, Rao AM, Dog A, Donaldson D, Gellin G, Dempsey RJ (1997) Regional brain polyamine levels in permanent focal cerebral ischemia. Brain Res 744:302–308
Bellomo R, Kellum JA, Ronco C (2012) Acute kidney injury. Lancet 380:756–766
Bonventre JV, Weinberg JM (2003) Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14:2199–2210
Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS (2002) Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am J Physiol Renal Physiol 282:F1140–F1149
Daemen MA, De Vries B, Buurman WA (2002) Apoptosis and inflammation in renal reperfusion injury. Transplantation 73:1693–1700
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149:1060–1072
Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, Fussi H, Deszcz L, Hartl R, Schraml E, Criollo A, Megalou E, Weiskopf D, Laun P, Heeren G, Breitenbach M, Grubeck-Loebenstein B, Herker E, Fahrenkrog B, Frohlich KU, Sinner F, Tavernarakis N, Minois N, Kroemer G, Madeo F (2009) Induction of autophagy by spermidine promotes longevity. Nat Cell Biol 11:1305–1314
Eisenberg T, Abdellatif M, Schroeder S, Primessnig U, Stekovic S, Pendl T, Harger A, Schipke J, Zimmermann A, Schmidt A, Tong M, Ruckenstuhl C, Dammbrueck C, Gross AS, Herbst V, Magnes C, Trausinger G, Narath S, Meinitzer A, Hu Z, Kirsch A, Eller K, Carmona-Gutierrez D, Buttner S, Pietrocola F, Knittelfelder O, Schrepfer E, Rockenfeller P, Simonini C, Rahn A, Horsch M, Moreth K, Beckers J, Fuchs H, Gailus-Durner V, Neff F, Janik D, Rathkolb B, Rozman J, De Angelis MH, Moustafa T, Haemmerle G, Mayr M, Willeit P, Von Frieling-Salewsky M, Pieske B, Scorrano L, Pieber T, Pechlaner R, Willeit J, Sigrist SJ, Linke WA, Muhlfeld C, Sadoshima J, Dengjel J, Kiechl S, Kroemer G, Sedej S, Madeo F (2016) Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med 22:1428–1438
Eltzschig HK, Eckle T (2011) Ischemia and reperfusion—from mechanism to translation. Nat Med 17:1391–1401
Frezza C, Cipolat S, Scorrano L (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2:287–295
Hyvonen MT, Sinervirta R, Grigorenko N, Khomutov AR, Vepsalainen J, Keinanen TA, Alhonen L (2010) alpha-Methylspermidine protects against carbon tetrachloride-induced hepatic and pancreatic damage. Amino Acids 38:575–581
Jamwal S, Kumar P (2016) Spermidine ameliorates 3-nitropropionic acid (3-NP)-induced striatal toxicity: possible role of oxidative stress, neuroinflammation, and neurotransmitters. Physiol Behav 155:180–187
Jiang M, Liu K, Luo J, Dong Z (2010) Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol 176:1181–1192
Kim J (2016) Poly(ADP-ribose) polymerase activation induces high mobility group box 1 release from proximal tubular cells during cisplatin nephrotoxicity. Physiol Res 65:333–340
Kim J, Padanilam BJ (2013) Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol 24:229–242
Kim J, Padanilam BJ (2015) Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int 87:350–358
Kim J, Kil IS, Seok YM, Yang ES, Kim DK, Lim DG, Park JW, Bonventre JV, Park KM (2006) Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J Biol Chem 281:20349–20356
Kim J, Kim KY, Jang HS, Yoshida T, Tsuchiya K, Nitta K, Park JW, Bonventre JV, Park KM (2009a) Role of cytosolic NADP+-dependent isocitrate dehydrogenase in ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 296:F622–F633
Kim J, Seok YM, Jung KJ, Park KM (2009b) Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol 297:F461–F470
Kim J, Long KE, Tang K, Padanilam BJ (2012) Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int 82:193–203
Kim J, Devalaraja-Narashimha K, Padanilam BJ (2015) TIGAR regulates glycolysis in ischemic kidney proximal tubules. Am J Physiol Renal Physiol 308:F298–F308
Kitada M, Igarashi K, Hirose S, Kitagawa H (1979) Inhibition by polyamines of lipid peroxide formation in rat liver microsomes. Biochem Biophys Res Commun 87:388–394
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87:99–163
Lee JS, Lim JY, Kim J (2015) Mechanical stretch induces angiotensinogen expression through PARP1 activation in kidney proximal tubular cells. In Vitro Cell Dev Biol Anim 51:72–78
Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S (2013) Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci USA 110:12024–12029
Lukkarainen J, Kauppinen RA, Koistinaho J, Halmekyto Alhonen LM, Janne J (1995) Cerebral energy metabolism and immediate early gene induction following severe incomplete ischaemia in transgenic mice overexpressing the human ornithine decarboxylase gene: evidence that putrescine is not neurotoxic in vivo. Eur J Neurosci 7:1840–1849
Lukkarinen JA, Kauppinen RA, Grohn OH, Oja JM, Sinervirta R, Jarvinen A, Alhonen LI, Janne J (1998) Neuroprotective role of ornithine decarboxylase activation in transient focal cerebral ischaemia: a study using ornithine decarboxylase-overexpressing transgenic rats. Eur J Neurosci 10:2046–2055
Marton LJ, Pegg AE (1995) Polyamines as targets for therapeutic intervention. Annu Rev Pharmacol Toxicol 35:55–91
Mason J, Torhorst J, Welsch J (1984) Role of the medullary perfusion defect in the pathogenesis of ischemic renal failure. Kidney Int 26:283–293
Megosh L, Gilmour SK, Rosson D, Soler AP, Blessing M, Sawicki JA, O’brien TG (1995) Increased frequency of spontaneous skin tumors in transgenic mice which overexpress ornithine decarboxylase. Cancer Res 55:4205–4209
Morselli E, Mariño G, Bennetzen MV, Eisenberg T, Megalou E, Schroeder S, Cabrera S, Bénit P, Rustin P, Criollo A (2011) Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J Cell Biol 192:615–629
Mozdzan M, Szemraj J, Rysz J, Stolarek RA, Nowak D (2006) Anti-oxidant activity of spermine and spermidine re-evaluated with oxidizing systems involving iron and copper ions. Int J Biochem Cell Biol 38:69–81
Okumura S, Teratani T, Fujimoto Y, Zhao X, Tsuruyama T, Masano Y, Kasahara N, Iida T, Yagi S, Uemura T, Kaido T, Uemoto S (2016) Oral administration of polyamines ameliorates liver ischemia/reperfusion injury and promotes liver regeneration in rats. Liver Transpl 22:1231–1244
Padanilam BJ (2003) Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol 284:F608–F627
Park S, Yoon SP, Kim J (2015) Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells. Anat Cell Biol 48:66–74
Pegg AE (2016) Functions of polyamines in mammals. J Biol Chem 291:14904–14912
Song H, Yoon SP, Kim J (2016) Poly(ADP-ribose) polymerase regulates glycolytic activity in kidney proximal tubule epithelial cells. Anat Cell Biol 49:79–87
Til H, Falke H, Prinsen M, Willems M (1997) Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats. Food Chem Toxicol 35:337–348
Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG (1978) Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int 14:31–49
Wang Z, Zahedi K, Barone S, Tehrani K, Rabb H, Matlin K, Casero RA, Soleimani M (2004) Overexpression of SSAT in kidney cells recapitulates various phenotypic aspects of kidney ischemia-reperfusion injury. J Am Soc Nephrol 15:1844–1852
Yoon SP, Kim J (2015) Poly(ADP-ribose) polymerase 1 activation links ischemic acute kidney injury to interstitial fibrosis. J Physiol Sci 65:105–111
Yoon SP, Kim J (2016) Poly(ADP-ribose) polymerase 1 contributes to oxidative stress through downregulation of sirtuin 3 during cisplatin nephrotoxicity. Anat Cell Biol 49:165–176
Yoon SP, Kim J (2017) Exogenous CGRP upregulates profibrogenic growth factors through PKC/JNK signaling pathway in kidney proximal tubular cells. Cell Biol Toxicol. doi:10.1007/s10565-017-9399-4
Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL (2002) Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297:259–263
Zahedi K, Wang Z, Barone S, Prada AE, Kelly CN, Casero RA, Yokota N, Porter CW, Rabb H, Soleimani M (2003) Expression of SSAT, a novel biomarker of tubular cell damage, increases in kidney ischemia-reperfusion injury. Am J Physiol Renal Physiol 284:F1046–F1055
Acknowledgements
The author thanks Youngsu Cho (JNU) for technical assistance with western blot and cell culture. This work was supported by a research grant from the Jeju National University Hospital Research Fund of Jeju National University in 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The author has no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Kim, J. Spermidine rescues proximal tubular cells from oxidative stress and necrosis after ischemic acute kidney injury. Arch. Pharm. Res. 40, 1197–1208 (2017). https://doi.org/10.1007/s12272-017-0957-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0957-3