Abstract
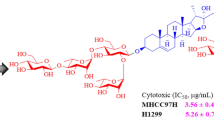
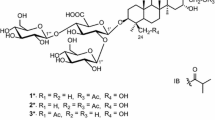
Four new ursane-type triterpenoid saponins, clinopoursaponins A–D (1–4), six new oleanane-type triterpenoid saponins, clinopodiside VII–XII (5–10), as well as eight known triterpene analogues (11–18), were isolated from the aerial parts of Clinopodium chinense (Benth.) O. Kuntze. The structures of the new compounds were determined based on extensive spectral analyses, including 1D (1H and 13C) and 2D NMR experiments (COSY, NOESY, HSQC, 2D TOCSY, HSQC-TOCSY and HMBC), HR-ESI-MS and chemical methods. Compounds 1–18 were evaluated for their protective effects against anoxia/reoxygenation-induced apoptosis in H9c2 cells and cytotoxicities against murine mammary carcinoma cell line 4T1. Compounds 8, 9 and 18 exhibited significant protective effects, while compound 1 exhibited cytotoxic activity with IC50 value of 7.4 μm compared to 7.6 μm for the positive control 10-hydroxycamptothecin.



Similar content being viewed by others
References
Aoshima H, Miyase T, Warashina T (2012) Caffeic acid oligomers with hyaluronidase inhibitory activity from Clinopodium gracile. Chem Pharm Bull 60:499–507
Begum S, Zehra SQ, Siddiqui BS (2008) Two new pentacyclic triterpenoids from Lantana camara Linn. Chem Pharm Bull 56:1317–1320
Chen JY, Chen JM, Wan CL, Shan J (1998) A New Anthraquinone from Clinopodium polycephalum glyceroyl-1,6,8-trihydroxy-3-methyl-9,10-dioxo-2-anthracene Carboxylate. Chin Chem Lett 9:143–144
Chen IH, Chang FR, Wu CC, Chen SL, Hsieh PW, Yen HF, Du YC, Wu YC (2006) Cytotoxic triterpenoids from the leaves of Microtropis fokienensis. J Nat Prod 69:1543–1546
Chinese Pharmacopoeia Commission (2015) Pharmacopoeia of the People’s Republic of China, vol 1. China Medical Science Press, Beijing, p 326
Farimani MM, Bahadori MB, Koulaei SA, Salehi P, Ebrahimi SN, Khavasi HR, Hamburger M (2015) New ursane triterpenoids from Salvia urmiensis Bunge: absolute configuration and anti-proliferative activity. Fitoterapia 106:1–6
Gao LM, Wei XM, Cheng DL (2003) Oleanane-triterpene saponins from Clinopodium urticifolium. Chin Chem Lett 14:1041–1044
Khedr AI, Ibrahim SR, Mohamed GA, Ahmed HE, Ahmad AS, Ramadan MA, El-Baky AE, Yamada K, Ross S (2016) A new ursane triterpenoids from Ficus pandurata and their binding affinity for human cannabinoid and opioid receptors. Arch Pharm Res 39:897–911
Liu ZC, Chen JY, Huang WD, Zeng Z, Yang YF, Zhu BH (2013) Ginsenoside Rb1 protects rat retinal ganglion cells against hypoxia and oxidative stress. Mol Med Rep 8:1397–1403
Miyase T, Matsushima Y (1997) Saikosaponin homologues from Clinopodium spp. the structures of clinoposaponins XII–XX. Chem Pharm Bull 45:1493–1497
Murata T, Sasaki K, Sato K, Yoshizaki F, Yamada H, Mutoh H, Umehara K, Miyase T, Warashina T, Aoshima H, Tabata H, Matsubara K (2009) Matrix metalloproteinase-2 inhibitors from Clinopodium chinense var. parviflorum. J Nat Prod 72:1379–1384
Shimizu K, Amagaya S, Ogihara Y (1985) New derivatives of saikosaponins. Chem Pharm Bull 33:3349–3355
Tian DN, Wu FH, Ma SC, Li D, Dai Y (2008) Studies on anti-hyperglycemic effect and its mechanism of Clinopodium chinense. Chin J Chin Mater Med 33:1313–1316
Wang SN, Ma GX, Zhong ML, Yu SC, Xu XD, Hu YX, Zhang YZ, Wei H, Yang JS (2013) Triterpene saponins from Tabellae Clinopodii. Fitoterapia 90:14–19
Wei XM, Cheng JK, Cheng DL, Gao LM (2004) Chemical constituents from Clinopodium urticifolium. J Chin Chem Soc 51:1043–1049
Yamamoto A, Miyase T, Ueno A, Maeda T (1993) Clinoposaponins I-V, new oleanane-triterpene saponins from Clinopodium gracile O. Kuntze. Chem Pharm Bull 41:1270–1274
Yang YL, Zhang XJ, Yu CT, Hao XJ, Jie JS, Zhou MJ, Zhang XH (2014) Smart nanorods for highly effective cancer theranostic applications. Adv Healthc Mater 3:906–915
Zhao LX, Tian MZ, Jin LJ, He XL, Shen P, Zhang XC, Yang JW (2011) Synthesis and characterization of derivatives of asiatic acid and primary study on anti-cancer activity. Chin J Org Chem 31:646–652
Zhong ML, Xu XD, Yu SC, Sun GL (2012) Advances in studies on medicinal plants in Clinopodium Linn. Chin J Chin Mater Med 43:820–828
Zhong ML, Wu HF, Zhang XP, Sun GL, Yu SC, Xu XD (2014) A new diterpene from Clinopodium chinense. Nat Prod Res 28:467–472
Zhu YD, Wu HF, Ma GX, Chen RC, Long HL, Zuo ZL, Luo Y, Zhu NL, Hou B, Xu XD (2016a) Clinoposides A-F: meroterpenoids with protective effects on H9c2 cardiomyocyte from Clinopodium Chinense. Rsc Adv 6:7260–7266
Zhu YD, Zhang JY, Li PF, Wu HF, Zhu NL, Jiang H, Lv CY, Wu LL, Ma ZX, Xu XD, Ma GX, Yang JS (2016b) Two new abietane diterpenoid glycosides from Clinopodium chinense. Nat Prod Res 30:1075–1080
Acknowledgements
This work was supported by Grants (Nos. 81173511 and 81374010) from the National Natural Sciences Foundation of China and Wenzhou Medical University Research Initiation Fund (No. QTJ16015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, YD., Hong, JY., Bao, FD. et al. Triterpenoid saponins from Clinopodium chinense (Benth.) O. Kuntze and their biological activity. Arch. Pharm. Res. 41, 1117–1130 (2018). https://doi.org/10.1007/s12272-017-0943-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0943-9