Abstract
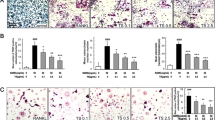
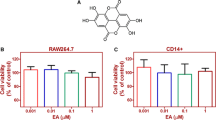
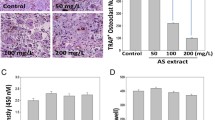
Magnolol, honokiol, and obovatol are well known bioactive constituents of the bark of Magnolia officinalis and have been reported to have beneficial effects in various diseases. We recently isolated a novel active compound, 4-O-methylhonokiol (4-O-MH) from the ethanol extract of M. officinalis, which was previously reported to have pharmacological effects including anti-inflammatory, anti-oxidative, and anti-aging activities. Here, we examined the pharmacological properties of 4-O-MH on osteoblast (bone-forming cells) and osteoclast (bone-resorbing cells) differentiation, and its underlying signaling pathways in primary cultured pre-osteoblasts and bone marrow macrophages. Our results showed that 4-O-MH did not affect cell viability in pre-osteoblasts and did not influence osteoblast differentiation and mineralized nodule formation, as assessed by alkaline phosphatase activity and Alizarin red staining. However, 4-O-MH significantly inhibited TRAP-positive multinuclear osteoclasts and F-actin ring formation during Receptor activator of NF-κB ligand (RANKL)-mediated osteoclastogenesis without cytotoxicity. In addition, 4-O-MH suppressed RANKL-induced critical factors (c-Fos, NF-ATc1, TRAP, and ITB3) for osteoclast differentiation and function. Furthermore, RANKL-mediated signaling, including ERK1/2, AKT, and NF-kB pathways was attenuated by 4-O-MH. Taken together, 4-O-MH has an inhibitory role in RANKL-mediated osteoclastogenesis but not osteoblast differentiation, and our findings also suggest that 4-O-MH is a potential therapeutic agent for bone-destructive diseases such as osteoporosis, alveolar bone resorption, and osteoarthritis.







Similar content being viewed by others
References
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Cairoli E, Zhukouskaya VV, Eller-Vainicher C, Chiodini I (2015) Perspectives on osteoporosis therapies. J Endocrinol Invest 38:303–311
Chang EJ, Ha J, Oerlemans F, Lee YJ, Lee SW, Ryu J, Kim HJ, Lee Y, Kim HM, Choi JY, Kim JY, Shin CS, Pak YK, Tanaka S, Wieringa B, Lee ZH, Kim HH (2008) Brain-type creatine kinase has a crucial role in osteoclast-mediated bone resorption. Nat Med 14:966–972
Endo I, Matsumoto T (2012) Update and perspectives of anabolic therapies for osteoporosis. Clin Calcium 22:327–333
Filvaroff E, Erlebacher A, Ye J, Gitelman SE, Lotz J, Heillman M, Derynck R (1999) Inhibition of TGF-beta receptor signaling in osteoblasts leads to decreased bone remodeling and increased trabecular bone mass. Development 126:4267–4279
Jung YY, Lee YJ, Choi DY, Hong JT (2014) Amelioration of cognitive dysfunction in APP/PS1 double transgenic mice by long-term treatment of 4-O-methylhonokiol. Biomol Ther (Seoul) 22:232–238
Khosla S, Riggs BL (2005) Pathophysiology of age-related bone loss and osteoporosis. Endocrinol Metab Clin North Am 34:1015–1030
Kim MB, Song Y, Hwang JK (2014) Kirenol stimulates osteoblast differentiation through activation of the BMP and Wnt/beta-catenin signaling pathways in MC3T3-E1 cells. Fitoterapia 98:59–65
Kwak HB, Lee BK, Oh J, Yeon JT, Choi SW, Cho HJ, Lee MS, Kim JJ, Bae JM, Kim SH, Kim HS (2010) Inhibition of osteoclast differentiation and bone resorption by rotenone, through down-regulation of RANKL-induced c-Fos and NFATc1 expression. Bone 46:724–731
Lee HS, Jung EY, Bae SH, Kwon KH, Kim JM, Suh HJ (2011a) Stimulation of osteoblastic differentiation and mineralization in MC3T3-E1 cells by yeast hydrolysate. Phytother Res 25:716–723
Lee YJ, Choi IS, Park MH, Lee YM, Song JK, Kim YH, Kim KH, Hwang DY, Jeong JH, Yun YP, Oh KW, Jung JK, Han SB, Hong JT (2011b) 4-O-methylhonokiol attenuates memory impairment in presenilin 2 mutant mice through reduction of oxidative damage and inactivation of astrocytes and the ERK pathway. Free Radic Biol Med 50:66–77
Lee YJ, Lee YM, Lee CK, Jung JK, Han SB, Hong JT (2011c) Therapeutic applications of compounds in the Magnolia family. Pharmacol Ther 130:157–176
Lee YJ, Choi DY, Choi IS, Kim KH, Kim YH, Kim HM, Lee K, Cho WG, Jung JK, Han SB, Han JY, Nam SY, Yun YW, Jeong JH, Oh KW, Hong JT (2012) Inhibitory effect of 4-O-methylhonokiol on lipopolysaccharide-induced neuroinflammation, amyloidogenesis and memory impairment via inhibition of nuclear factor-kappaB in vitro and in vivo models. J Neuroinflammation 9:35
Marie PJ, Kassem M (2011) Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. Eur J Endocrinol 165:1–10
Matsuo K, Galson DL, Zhao C, Peng L, Laplace C, Wang KZ, Bachler MA, Amano H, Aburatani H, Ishikawa H, Wagner EF (2004) Nuclear factor of activated T-cells (NFAT) rescues osteoclastogenesis in precursors lacking c-Fos. J Biol Chem 279:26475–26480
Monje P, Hernandez-Losa J, Lyons RJ, Castellone MD, Gutkind JS (2005) Regulation of the transcriptional activity of c-Fos by ERK. A novel role for the prolyl isomerase PIN1. J Biol Chem 280:35081–35084
Oh JH, Kang LL, Ban JO, Kim YH, Kim KH, Han SB, Hong JT (2009) Anti-inflammatory effect of 4-O-methylhonokiol, compound isolated from Magnolia officinalis through inhibition of NF-kappaB. Chem Biol Interact 180:506–514
Oh JH, Ban JO, Cho MC, Jo M, Jung JK, Ahn B, Yoon DY, Han SB, Hong JT (2012) 4-O-methylhonokiol inhibits colon tumor growth via p21-mediated suppression of NF-kappaB activity. J Nutr Biochem 23:706–715
Riggs BL, Hartmann LC (2003) Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med 348:618–629
Riggs BL, Parfitt AM (2005) Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 20:177–184
Takayanagi H (2009) Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol 5:667–676
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289:1504–1508
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649
Vondracek SF, Minne P, McDermott MT (2008) Clinical challenges in the management of osteoporosis. Clin Interv Aging 3:315–329
Whitmarsh AJ, Davis RJ (1996) Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med (Berl) 74:589–607
Yun HM, Kim S, Kim HJ, Kostenis E, Kim JI, Seong JY, Baik JH, Rhim H (2007) The novel cellular mechanism of human 5-HT6 receptor through an interaction with Fyn. J Biol Chem 282:5496–5505
Yun HM, Ban JO, Park KR, Lee CK, Jeong HS, Han SB, Hong JT (2014) Potential therapeutic effects of functionally active compounds isolated from garlic. Pharmacol Ther 142:183–195
Yun HM, Park KR, Quang TH, Oh H, Hong JT, Kim YC, Kim EC (2015) 2,4,5-Trimethoxyldalbergiquinol promotes osteoblastic differentiation and mineralization via the BMP and Wnt/beta-catenin pathway. Cell Death Dis 6:e1819
Yun HM, Park KR, Hong JT, Kim EC (2016) Peripheral serotonin-mediated system suppresses bone development and regeneration via serotonin 6 G-protein-coupled receptor. Sci Rep 6:30985
Zanotti S, Canalis E (2015) Activation of Nfatc2 in Osteoblasts Causes Osteopenia. J Cell Physiol 230:1689–1695
Acknowledgements
This work was supported by a grant from Kyung Hee University in 2016 (KHU-20160546) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (MRC, 20080062275; 2015R1D1A1A01059240).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Park, KR., Kim, JY., Kim, EC. et al. RANKL-induced osteoclastogenesis is suppressed by 4-O-methylhonokiol in bone marrow-derived macrophages. Arch. Pharm. Res. 40, 933–942 (2017). https://doi.org/10.1007/s12272-017-0932-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-017-0932-z