Abstract
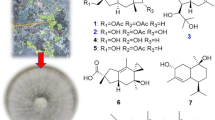
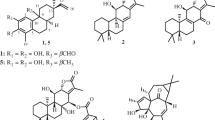
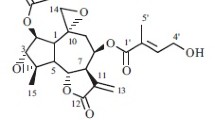
A new rearranged eudesmane sesquiterpene, named eudeglaucone (1), and five known sesquiterpenes including (+)-faurinone (2) and four eudesmane-type sesquiterpenes (3–6), were isolated from the twigs of Lindera glauca (Sieb. et Zucc.) Blume. The structure of 1 was elucidated by a combination of extensive spectroscopic analyses, including extensive 2D NMR (1H-1H COSY, HMQC, HMBC, and NOESY) and HR-MS. Compound 1 was a relatively rare rearranged eudesmane sesquiterpene in terpenoids. All isolates were evaluated for their antiproliferative activities against four human tumor cell lines (A549, SK-OV-3, SK-MEL-2, and HCT-15). Compounds 3 and 6 showed significant cytotoxicity against SK-MEL-2 and HCT-15 cell lines with IC50 values ranging from 9.98 to 12.20 μM. We also investigated the anti-neuroinflammatory activities of the isolates (1–6) in the lipopolysaccharide (LPS)-stimulated murine microglia BV-2 cell line by measuring nitric oxide (NO) levels. All isolates significantly inhibited NO production with IC50 values of 3.67–26.48 μM without inducing cell toxicity.


Similar content being viewed by others
References
Bos R, Hendriks H, Kloosterman J, Sipma G (1983) A structure of faurinone, a sesquiterpene ketone isolated from Valeriana officinalis. Phytochemistry 22:1505–1506
Ceccherelli P, Curini M, Marcotullio MC, Menghini A (1985) Sesquiterpene acids from Dittrichia viscosa. Phytochemistry 24:2987–2989
Chang YC, Chang FR, Wu YC (2000) The constituents of Lindera glauca. J Chin Chem Soc 47:373–380
Chang YC, Chen CY, Chang FR, Wu YC (2001) Alkaloids from Lindera glauca. J Chin Chem Soc 48:811–815
Cheng XR, Zhang SD, Wang CH, Ren J, Qin JJ, Tang X, Shen YH, Yan SK, Jin HZ, Zhang WD (2013) Bioactive eudesmane and germacrane derivatives from Inula wissmanniana Hand.-Mazz. Phytochemistry 96:214–222
Chung BS, Shin MK (1990) Dictionary of Korean folk medicine. Young Lim, Seoul
Garcez FR, Garcez WS, Hamerski L, Miranda ACDM (2010) Eudesmane and rearranged eudesmane sesquiterpenes from Nectandra cissiflora. Quim Nova 33:1739–1742
Huh GW, Park JH, Shrestha S, Lee YH, Ahn EM, Kang HC, Baek NI (2011) Sterols from Lindera glauca Blume stem wood. J Appl Biol Chem 54:309–312
Huh GW, Park JH, Shrestha S, Lee YH, Ahn EM, Kang HC, Kim YB, Baek NI (2012) New diarylpropanoids from Lindera glauca Bl Heartwood. Holzforschung 66:585–590
Jiang B, Wang WJ, Li MP, Huang XJ, Huang F, Gao H, Sun PH, He MF, Jiang ZJ, Zhang XQ, Ye WC (2013) New eudesmane sesquiterpenes from Alpinia oxyphylla and determination of their inhibitory effects on microglia. Bioorg Med Chem Lett 23:3879–3883
Kim KH, Choi SU, Kim YC, Lee KR (2011) Tirucallane triterpenoids from Cornus walteri. J Nat Prod 74:54–59
Kim KH, Moon E, Ha SK, Suh WS, Kim HK, Kim SY, Choi SU, Lee KR (2014) Bioactive lignan constituents from the twigs of Lindera glauca. Chem Pharm Bull 62:1136–1140
Kim KH, Clardy J, Senger D, Cao S (2015a) Chakyunglupulins A and B, two novel 4,8,8-trimethylcyclooct-2-enone derivatives from Barleria lupulina. Tetrahedron Lett 56:2732–2734
Kim S, Oh MH, Kim BS, Kim WI, Cho HS, Park BY, Park C, Shin GW, Kwon J (2015b) Upregulation of heme oxygenase-1 by ginsenoside Ro attenuates lipopolysaccharide-induced inflammation in macrophage cells. J Ginseng Res 39:365–370
Ko W, Sohn JH, Kim YC, Oh H (2015) Viridicatol from marine-derived fungal strain Penicillium sp. SF-5295 exerts anti-inflammatory effects through inhibiting NF-κB signaling pathway on lipopolysaccharide-induced RAW264.7 and BV2 cells. Nat Prod Sci 21:240–247
Lee TB (1998) Coloured flora of Korea. Hyangmunsa, Seoul
Leon LG, Donadel OJ, Tonn CE, Padron JM (2009) Tessaric acid derivatives induce G2/M cell cycle arrest in human solid tumor cell lines. Bioorg Med Chem 17:6251–6256
McGeer PL, McGeer EG (1995) The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Rev 21:195–218
Nie XP, Yao F, Yue XD, Li GS, Dai SJ (2014) New eudesmane-type sesquiterpenoid from Solanum lyratum with cytotoxic activity. Nat Prod Res 28:641–645
Nii H, Furukawa K, Iwakiri M, Kubota T (1983) A new sesquiterpene carboxylic acid from Lindera glauca (Sieb. et Zucc.) Blume. Nippon Nogei Kagaku Kaishi 57:725–732
Oksuz S, Topcu G (1991) A eudesmanolide and other constituents from Inula graveolens. Phytochemistry 31:195–197
Park SY, Neupane GP, Lee SO, Lee JS, Kim MY, Kim SY, Park BC, Park YJ, Kim JA (2014) Protective effects of Pogostemon cablin Bentham water extract on inflammatory cytokine expression in TNBS-induced colitis in rats. Arch Pharm Res 37:253–262
Seki K, Sasaki T, Haga K, Kaneko R (1994) Two methoxybutanolides from Lindera glauca. Phytochemistry 36:949–951
Seki K, Sasaki T, Wano S, Haga K, Kaneko R (1995) Linderanolides and isolinderanolides, ten butanolides from Lindera glauca. Phytochemistry 40:1175–1181
Song JH, Choi HJ, Song HH, Hong EH, Lee BR, Oh SR, Choi K, Yeo SG, Lee YP, Cho S, Ko HJ (2014) Antiviral activity of ginsenosides against coxsackievirus B3, enterovirus 71, and human rhinovirus 3. J Ginseng Res 38:173–179
Suh WS, Kim KH, Kim HK, Choi SU, Lee KR (2015) Three new lignan derivatives from Lindera glauca (Siebold et Zucc.) Blume. Helv Chim Acta 98:1087–1094
Tian SH, Chai XY, Zan K, Zeng KW, Tu PF (2013) Three new eudesmane sesquiterpenes from Artemisia vestita. Chin Chem Lett 24:797–800
Todorova MN, Tsankova ET (1999) New sesquiterpenoids from Achillea clypeolata. Phytochemistry 52:1515–1518
Wang S, Sun J, Zeng K, Chen X, Zhou W, Zhang C, Jin H, Jiang Y, Tu P (2014) Sesquiterpenes from Artemisia argyi: absolute configurations and biological activities. Eur J Org Chem 2014:973–983
Watanabe Y, Mihara R, Mitsunaga T, Yoshimura T (2005) Termite repellent sesquiterpenoids from Callitris glaucophylla heartwood. J Wood Sci 51:514–519
Wilms H, Zecca L, Rosenstiel P, Sievers J, Deuschl G, Lucius R (2007) Inflammation in Parkinson’s diseases and other neurodegenerative diseases: cause and therapeutic implications. Curr Pharm Des 13:1925–1928
Yang Y, Lee J, Rhee MH, Yu T, Baek KS, Sung NY, Kim Y, Yoon KY, Kim JH, Kwak YS, Hong S, Kim JH, Cho JY (2015) Molecular mechanism of protopanaxadiol saponin fraction-mediated anti-inflammatory actions. J Ginseng Res 39:61–68
Zhang L, Lin HQ, Li GS, Yue XD, Dai SJ (2015) New sesquiterpenoid derivatives from Solanum septemlobum with cytotoxicities. Nat Prod Res 29:1889–1893
Zhao J, Wu J, Yan F (2014) A new sesquiterpenoid from the rhizomes of Homalomena occulta. Nat Prod Res 28:1669–1673
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2015R1C1A1A02037383) and by the Ministry of Education (NRF-2012R1A5A2A28671860).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, J.S., Baek, J., Park, H.B. et al. A new rearranged eudesmane sesquiterpene and bioactive sesquiterpenes from the twigs of Lindera glauca (Sieb. et Zucc.) Blume. Arch. Pharm. Res. 39, 1628–1634 (2016). https://doi.org/10.1007/s12272-016-0838-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0838-1