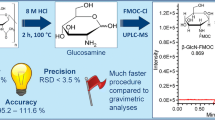
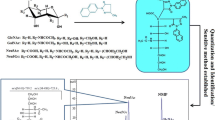
Abstract
β-N-acetylglucosamine (β-AG) is a monosaccharide distributed widely in living organisms with various pivotal roles. The presence of particulates and impurities can affect the safety and efficacy of a product for its intended duration of use. Thus, the current study was carried out to identify and quantify the potentially-harmful process related impurities; namely α-N,6-diacetylglucosamine (α-DAG) and α-N-acetylglucosamine (α-AG), derived from the chemical and enzymatic synthesis of β-AG. The impurities were characterized using a high resolution mass spectrometry, a nuclear magnetic resonance spectroscopy, and liquid chromatography-tandem mass spectrometry (LC/MS/MS). The developed method showed a good linearity (R 2 ≥ 0.998), satisfactory precision (≤6.1 % relative standard deviation), intra- and inter-day accuracy (88.20–97.50 %), extraction recovery (89.30–110.50 %), matrix effect (89.70–105.20 %), and stability (92.70–101.60 %). The method was successfully applied to determine the level of α-DAG that was 3.04 and 0.07 % of the total β-AG, following chemical and enzymatic methods, respectively. It can be concluded that the enzymatic rather than the chemical method is more efficient for the synthesis of β-AG. Characterization of impurities heeds the signal for acquiring and evaluating data that establishes biological safety.



Similar content being viewed by others
References
Chen JK, Shen CR, Liu CL (2010) N-Acetylglucosamine: production and applications. Mar Drugs 8:2493–2516
Eeckhaut AV, Lanckmans K, Sarre S, Smolders I, Michotte Y (2009) Validation of bioanalytical LC–MS/MS assays: evaluation of matrix effects. J Chromatogr B 877:2198–2207
EMEA (2009) http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/12/WC500018062.pdf
Gooday GW (1990) The ecology of chitin degradation. Adv Microb Ecol 11:387–430
Görög S, Babják M, Balogh G, Brlik J, Csehi A, Dravecz F, Gasdag M, Horváth P, Laukó A, Varga K (1997) Drug impurity profiling strategies. Talanta 44:1517–1526
Haynes CA, Aloise P, Creagh AL (1999) Process for producing N-acetyl-d-glycosamine. U.S. Patent 5,998,173. https://www.google.com/patents/US5998173?dq=inassignee:%22The+University+Of+Bristish+Columbia%22&hl=en&sa=X&ved=0ahUKEwioh8X2p9_JAhWj4KYKHSYZDm8Q6AEIJDAB
Hoff JE (1963) Determination of N-acetylglucosamine-1-phosphate and N-acetylglucosamine in milk. J Dairy Sci 46:573–574
Jollès P, Muzzarelli RAA (eds) (1999) Chitin and chitinases. Birkhauser Verlag publishers, Basel
Kohn P, Winzler RJ, Hoffmann RC (1962) Metabolism of d-glucosamine and N-acetyl-d-glucosamine in the intact rat. J Biol Chem 237:304–308
Kojima H, Shimizu T, Suigita M, Itonori S, Fujita N, Ito M (2011) Biochemical studies on sphingolipids of Artemia franciscana: novel neutral glycosphingolipids. J Lipid Res 52:308–317
Liu CL, Tsai CC, Lin SC, Wang LI, Hsu CI, Hwang MJ, Lin JY (2000) Primary structure and function analysis of the abrus precatorius agglutinin a chain by site-directed mutagenesis. J Biol Chem 257:1897–1901
Mast BA, Flood LC, Haynes JH, DePalma RL, Cohen IK, Dieqelmann RF, Krummel TM (1991) Hyaluronic acid is a major component of the matrix of fetal rabbit skin and wounds: implications for healing by regeneration. Matrix 11:63–68
Onarheim H, Brofeldt BT, Gunther RA (1996) Markedly increased lymphatic removal of hyaluronan from skin after major thermal injury. Burns 11:212–216
Pretsch E, Bühlmann P, Affolter C (2000) Structure determination of organic compounds—tables of spectral data. Springer, New York, p 153
Roseman S, Ludoweig J (1954) N-Acetylation of hexosamines. J Am Chem Soc 76:301–302
Sashiwa H, Fujishima S, Yamano N, Kawasaki N, Nakayama A, Muraki E, Sukwattanasinitt M, Pichyangkura R, Aiba S (2003) Enzymatic production of N-acetyl-d-glucosamine from chitin. Degradation study of N-acetylchitooligosaccharide and the effect of mixing of crude enzymes. Carbohydr Polym 51:391–395
Sato M, Ishikawa O, Yokoyama Y, Kondo A, Miyachi Y (1996) Focal dermal hypoplasia (Goltz syndrome): a decreased accumulation of hyaluronic acid in three-dimensional culture. Acta Dermato-Venereol 76:365–367
Shibata K, Tsubouchi R (2008) Clinical effects of N-acetylglucosamine supplementation on dry skin. Aesthet Dermatol 18:91–99
Shin YC, Jun YJ, Yoon YC, Kim YS (2006) Chitinase-producing trichoderma viride A G C C-M41strain, chitinases purified therefrom and a method for producing N-acetylglucosamine using the chitinases. Republic of Korea. Patent 10-2006-0070224. http://kpat.kipris.or.kr/kpat/biblioa.do?method=biblioFrame
Tanaka T, Fujiwara S, Nishikori S, Fukui T, Takagi M, Imanaka TA (1999) Unique chitinase with dual active sites and triple substrate binding sites from the hyperthermophilic archaeon pyrococcus kodakaraensis KOD1. Appl Environ Microbiol 65:5338–5344
U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research. Guidance for industry, bioanalytical method validation; FDA (2001)
Wu AM, Wu JH, Liu JH, Chen YY, Singha B, Chow LP, Lin JY (2009) Roles of mammalian structural units, ligand cluster and polyvalency in the abrus precatorius agglutinin and glycoprotein recognition process. Mol Immunol 16:3427–3437
Zhan WS (2007) Process for preparing refined N-acetyl-d-aminoglucose. PAT—CN1907993
Acknowledgments
This work was supported by Amicogen, INC., Jinju, Republic of Korea.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflict of interest.
Additional information
Yi Soo Kim and Sung Joong Lee have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kim, Y.S., Lee, S.J., Choi, J.Y. et al. Determination of process-related impurities in N-acetylglucosamine prepared by chemical and enzymatic methods: structural elucidation and quantification. Arch. Pharm. Res. 39, 937–945 (2016). https://doi.org/10.1007/s12272-016-0755-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0755-3