Abstract
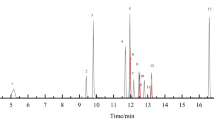
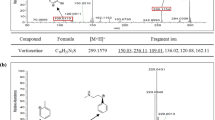
Recently, the number of the cases in which weight loss products have been sold with illegal adulterants has increased, as awareness of the problems of obesity grows. In this study, we developed simultaneous analysis methods to rapidly and accurately identify ingredients illegally mixed with foods and dietary supplements. Twenty-three anti-obesity drugs in foods and dietary supplements were determined by developed and validated UPLC and LC-MS/MS methods. The UPLC method were validated for the LOD and LOQ in the ranges 0.05–3.0 and 0.2–10.0 μg/mL, respectively. The determination coefficient was over 0.999, precision was <6.2 %, and the accuracy was 80.8–103.9 %. The mean recoveries ranged from 80.3 to 109.3 % and RSD of stability was less than 2.1 %. The determination of the 23 anti-obesity drugs was accomplished by electrospray ionization LC-MS/MS using multiple reaction monitoring (MRM). The LODs and LOQs were in the ranges 0.03–7.5 and 0.09–30.0 ng/mL, respectively. The LC-MS/MS method was validated for linearity (r2 > 0.99), precision (RSD < 10.7 %), accuracy (94.1–109.1 %), recovery (80.5–113.5 %), and the RSD of stability was <7.8 %. Using the newly developed and validated method, 193 samples were tested, and 55 were found to be adulterated.




Similar content being viewed by others
References
Deconinck, E., K. Verlinde, P. Courselle, and J.O. De Beer. 2012. A validated ultra high pressure liquid chromatographic method for the characterisation of confiscated illegal slimming products containing anorexics. Journal of Pharmaceutical and Biomedical Analysis 59: 38–43.
Dunn, J.D., C.M. Gryniewicz-Ruzicka, J.F. Kauffman, B.J. Westenberger, and L.F. Buhse. 2011. Using a portable ion mobility spectrometer to screen dietary supplements for sibutramine. Journal of Pharmaceutical and Biomedical Analysis 54: 469–474.
European Commission Decision No. 657/2002. 2002. Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Official Journal of the European Union L221: 8–36.
Halpern, A., and M.C. Mancini. 2003. Treatment of obesity: An update on anti-obesity medications. Obesity Reviews 4: 25–42.
Heal, D.J., J. Gosden, and S.L. Smith. 2012. What is the prognosis for new centrally-acting anti-obesity drugs? Neuropharmacology 63: 132–146.
Huang, Z., S. Xiao, D. Luo, B. Chen, and S. Yao. 2008. Simultaneous determination of sibutramine and N-di-desmethylsibutramine in dietary supplements for weight control by HPLC—ESI-MS. Journal of Chromatographic Science 46: 707–711.
ICH Q2 (R1) (2005). Validation of analytical procedures: Text and methodology. In International conference on harmonization of technical requirements for the registration of pharmaceutical for human use.
James, W.P. 2008. The epidemiology of obesity: The size of the problem. Journal of Internal Medicine 263: 336–352.
Kerrigan, S., and T. Lindsey. 2005. Fatal caffeine overdose: Two case reports. Forensic Science International 1153: 67–69.
Kim, H.J., J.H. Lee, H.J. Park, S.H. Cho, S. Cho, and W.S. Kim. 2014. Monitoring of 29 weight loss compounds in foods and dietary supplements by LC-MS/MS. Food Additives and Contaminants: Part A 31: 777–783.
Kim, J.W., S.J. Kweon, S.K. Park, J.Y. Kim, J.H. Lee, et al. 2013. Isolation and identification of a sibutramine analogue adulterated in slimming dietary supplements. Food Additives and Contaminants: Part A 30: 1221–1229.
Kim, S.H., J. Lee, T. Yoon, J. Choi, D. Chio, D. Kim, and S.W. Kwon. 2009. Simultaneous determination of anti-diabetes/anti-obesity drugs by LC/PDA, and targeted analysis of sibutramine analog in dietary supplements by LC/MS/MS. Biomedical Chromatography 23: 1259–1265.
Kiortsis, D.N. 2013. A review of the metabolic effects of controlled-release phentermine/topiramate. Hormones 12: 507–516.
Kokoletsi, M.X., S. Kafkala, and M. Tsiaganis. 2005. A novel gradient HPLC method for simultaneous determination of ranitidine, methylparaben and propylparaben in oral liquid pharmaceutical formulation. Journal of Pharmaceutical and Biomedical Analysis 38: 763–767.
Kong, W.J., T.L. Whao, X.H. Xiao, C. Jin, and Z.L. Li. 2009. Quantitative and chemical fingerprint analysis for quality control of Rhizoma Coptidischinensis based on UPLC-PDA combined with chemometrics methods. Phytomedicine 16: 950–959.
Li, Y., H. Zhang, J. Hu, F. Xue, Y. Li, and C. Sun. 2012. A GC-EI-MS-MS method for simultaneous determination of seven adulterants in slimming functional foods. Journal of Chromatographic Science 50: 928–933.
Padwal, R.S., and S.R. Majumdar. 2007. Drug treatments for obesity: orlistat, sibutramine, and rimonabant. Lancet 369: 71–77.
Shi, Y., C. Sun, B. Gao, and A. Sun. 2011. Development of a liquid chromatography tandem mass spectrometry method for simultaneous determination of eight adulterants in slimming functional foods. Journal of Chromatography A 1218: 7655–7662.
Song, F., D. Monroe, A. El-Demerdash, and C. Palmer. 2014. Screening for multiple weight loss and related drugs in dietary supplement materials by flow injection tandem mass spectrometry and their confirmation by liquid chromatography tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis 88: 136–143.
Tang, M.H.Y., S.P.L. Chen, S.W. Ng, A.Y.W. Chan, and T.W.L. Mak. 2011. Case series on a diversity of illicit weight-reducing agents: from well known to the unexpected. British Journal of Clinical Pharmacology 71: 250–253.
Yoon, K.H., J.H. Lee, J.W. Kim, J.H. Cho, Y.H. Choi, S.H. Ko, et al. 2006. Epidemic obesity and type 2 diabetes in Asia. Lancet 368: 1681–1688.
Zhang, X.D., X.Y. Fu, J. Chen, X.Y. Wu, Z.L. Gu, and G.H. Zhou. 2012. A HPLC-DAD spectrum library for rapidly detecting 10 anti-obesity chemicals illegally adulterated in traditional Chinese medicines and healthy foods. Chinese Journal of New Drugs 21(02): 195–201.
Acknowledgments
This research was supported by a Grant (12181MFDS705) from the Ministry of Food and Drug Safety (MFDS) in the Republic of Korea in 2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jung Yeon Kim and Hyoung Joon Park have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, J.Y., Park, H.J., Kim, J.W. et al. Development and validation of UPLC and LC-MS/MS methods for the simultaneous determination of anti-obesity drugs in foods and dietary supplements. Arch. Pharm. Res. 39, 103–114 (2016). https://doi.org/10.1007/s12272-015-0665-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-015-0665-9