Abstract
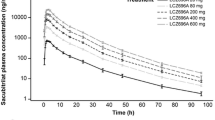
A substituted benzamide, amisulpride is an atypical antipsychotic and a specific antagonist for dopamine D2 and D3 receptors. The prandial effect on amisulpride absorption remains unclear, therefore, this study was designed to investigate the effect of food on the systemic exposure to amisulpride in healthy volunteers. The study was a randomized, two-way crossed trial in which a single oral dose of amisulpride was administered on two occasions, with 7-days washout period between each drug administration. The volunteers were randomly divided into two groups and received amisulpride (50 mg) with Korean traditional food or under fasting state. Blood was serially taken, and the plasma amisulpride concentrations were measured by LC/MS/MS. At fasting state, amisulpride reached the first peak (37.1 ± 13.3 ng/ml) at ~2.3 h, and decreased down to 19.4 ± 4.3 ng/ml until 3.5 h, and then again went up to the second peak (25.3 ± 5.8 ng/ml) at 5 h followed by a slow decay with 10.6 h of half-life. In contrast, no double peaks were shown when the drug was given with meal. The maximum concentration of amisulpride (56.0 ± 12.7 ng/ml) was increased by a 1.5-fold compared with that under fasting (p > 0.05), and the time to peak shortened a little (1.7 ± 0.6 h).


Similar content being viewed by others
References
Barclay, L. 2002. Aisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropsychopharmacology 27: 1071–1081.
Bergemanna, N., J. Kopitzb, K.R. Kressa, and A. Fricka. 2004. Plasma amisulpride levels in schizophrenia or schizopaffective disorder. European Neuropsychopharmacology 14: 245–250.
Chen, N., C. Kasserra, J. Reyes, L. Liu, and H. Lau. 2012. Single-dose pharmacokinetics of lenalidomide in healthy volunteers: dose proportionality, food effect, and racial sensitivity. Cancer Chemotherapy and Pharmacology 70: 717–725.
Gschwend, M.H., P. Arnold, J. Ring, and W. Martin. 2006. Selective and sensitive determination of amisulpride in human plasma by liquid chromatography-tandem mass spectrometry with positive electrospray ionisation and multiple reaction monitoring. Journal of Chromatography B 831: 132–139.
Hamon-Vilcot, B., S. Chaufour, C. Deschamps, M. Canal, I. Zieleniuk, P. Ahtoy, P. Chretien, P. Rosenzweig, A. Nasr, and F. Piette. 1998. Safety and pharmacokinetics of a single oral dose of amisulpride in healthy elderly volunteers. European Journal of Clinical Pharmacology 54: 405–409.
Kang, W., K. Kim, E.Y. Kim, K. Kwon, J.S. Bang, and Y.R. Yoon. 2008. Effect of food on systemic exposure to niflumic acid following postprandial administration of talniflumate. European Journal of Clinical Pharmacology 64: 1027–1030.
Kim, E.Y., and W. Kang. 2011. Contribution of pH to systemic exposure of niflumic acid following oral administration of talniflumate. European Journal of Clinical Pharmacology 67: 425–428.
Lim, H.K., S.J. Kim, C.U. Pae, C. Lee, and C.U. Lee. 2007. Comparison of amisulpride and risperidone in the treatment of psychosis in patients with dementia of the Alzheimer’s type. Journal of Korean Geriatric Psychiatry 11: 35–39.
Malavasi, B., M. Locatelli, M. Ripamonti, and V. Ascalone. 1996. Determination of amisulpride, a new benzamide derivative, in human plasma and urine by liquid–liquid extraction or solid-phase extraction in combination with high-performance liquid chromatography and fluorescence detection. application to pharmacokinetics. Journal of Chromatography B 676: 107–115.
Müller, M.J., F.X. Eich, B. Regenbogen, J. Sachse, S. Härtter, and C. Hiemke. 2009. Amisulpride doses and plasma levels in different age groups of patients with schizophrenia or schizoaffective disorder. Journal of Psychopharmacology 23: 278–286.
Nuss, P., M. Hummer, and C. Tessier. 2007. The use of amisulpride in the treatment of acute psychosis. Therapeutics and Clinical Risk Management 3: 3–11.
Rosenzweig, P., M. Canal, A. Patat, L. Bergougnan, I. Zieleniuk, and G. Bianchetti. 2002. A review of the pharmacokinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers. Human Psychopharmacology 17: 1–13.
Won, C.S., N.H. Oberlies, and M.F. Paine. 2012. Mechanisms underlying food-drug interactions: inhibition of intestinal metabolism and transport. Pharmacology & Therapeutics 136: 186–201.
Yun, H.Y., M.S. Baek, and K.I. Kwon. 2006a. The effect of food on absorption of drug in the gastrointestinal tract. Korean Journal of Clinical Pharmacy 16: 147–154.
Yun, H.Y., E.J. Lee, S.Y. Chung, S.O. Choi, H.K. Kim, J.T. Kwon, W. Kang, and K.I. Kwon. 2006b. The effects of food on the bioavailability of fenofibrate administered orally via sustained-release capsules in humans. Clinical Pharmacokinetics 45: 425–432.
Acknowledgments
This study was supported by Yeungnam University research grant in 2010.
Conflict of interests
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yoo-Jung Jang and Tae Cheon Jeong have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Jang, YJ., Jeong, T.C., Noh, K. et al. Prandial effect on the systemic exposure of amisulpride. Arch. Pharm. Res. 37, 1325–1328 (2014). https://doi.org/10.1007/s12272-014-0331-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-014-0331-7