Abstract
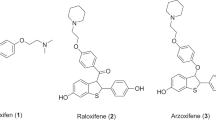
We have designed the cyclopropane analog of stilbene as subtype-selective ligands for estrogen receptor based on the bioisosterism that cyclopropane could act as alkene bioisoster. Three cyclopropane analogs were prepared efficiently starting from 4-benzyloxybenzaldehyde, and evaluated for their binding to estrogen receptors ERα and ERβ. These cyclopropane analogs were also found to be full agonists in estrogen receptor-mediated gene transcription assay. Compared to the stilbene analogs such as tamoxifen and raloxifene, the three cyclopropane analogs showed lower binding affinity for estrogen receptor, but higher subtype selectivity for ERα. The structure–activity relationship revealed from this study might provide clues for improving subtype selectivity for ERα.



Similar content being viewed by others
References
Ali, S., L. Buluwela, and R.C. Coombes. 2011. Antiestrogens and their therapeutic applications in breast cancer and other diseases. Annual Review of Medicine 62: 217–232.
Carpita, A., A. Ribecai, R. Rossi, and P. Stabile. 2002. Synthesis of the racemic forms of carbon–carbon double bond locked analogues of strobilurins which are characterized by a 2-arylcyclopropane ring cis-substituted at C-1 by the methyl (E)-3-methoxypropenoate unit. Tetrahedron 58: 3673–3680.
Catherino, W.H., and V.C. Jordan. 1995. Increasing the number of tandem estrogen response elements increases the estrogenic activity of a tamoxifen analogue. Cancer Letters 92: 39–47.
Chen, H.Y., K.D. Dykstra, E.T. Birzin, K. Frisch, W. Chan, Y.T. Yang, R.T. Mosley, F. DiNinno, S.P. Rohrer, J.M. Schaeffer, and M.L. Hammond. 2004. Estrogen receptor ligands. Part 1: The discovery of flavonoids with subtype selectivity. Bioorganic & Medicinal Chemistry Letters 14: 1417–1421.
Chlebowski, R.T., and G.L. Anderson. 2012. Changing concepts: Menopausal hormone therapy and breast cancer. Journal of the National Cancer Institute 104(7): 517–527.
Dadiboyena, S. 2012. Recent advances in the synthesis of raloxifene: A selective estrogen receptor modulator. European Journal of Medicinal Chemistry 51: 17–34.
Day, B.W., R.A. Magarian, P.T. Jain, J.T. Pento, G.K. Mousissian, and K.L. Meyer. 1991. Synthesis and biological evaluation of a series of l,l-dichloro-2,2,3-triarylcyclopropanes as pure antiestrogens. Journal of Medicinal Chemistry 34: 842–851.
Furukawa, J., N. Kawabata, and J. Nishimura. 1966. A novel route to cyclopropanes from olefins. Tetrahedron Letters 28: 3353–3354.
Lagu, B., R. Lebedev, B. Pio, M. Yang, and P. Pelton. 2007. Dihydro-[1H]-quinolin-2-ones as retinoid X receptor (RXR) agonists for potential treatment of dyslipidemia. Bioorganic & Medicinal Chemistry Letters 17: 3491–3496.
Nilsson, S., and J.-A. Gustafsson. 2002. Biological role of estrogen and estrogen receptors. Critical Reviews in Biochemistry and Molecular Biology 37(1): 1–28.
Overacre, L.B., and R.A. Magarian. 1998. Synthesis and biological evaluation of cyclopropyl analogs of the antiestrogen MER 25. Bioorganic Chemistry 26: 15–31.
Obourn, J.D., N.J. Koszewski, and A.C. Notides. 1993. Hormone- and DNA-binding mechanisms of the recombinant human estrogen receptor. Biochemistry 32: 6229–6236.
Pickar, J.H., T. MacNeil, and K. Ohleth. 2010. SERMs: Progress and future perspectives. Maturitas 67: 129–138.
Pike, A.C., A.M. Brzozowski, R.E. Hubbard, T. Bonn, A.G. Thorsell, O. Engstrom, J. Ljunggren, J.A. Gustafsson, and M. Carlquist. 1999. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO Journal 18: 4608–4618.
Rogers, M.T., and J.D. Roberts. 1946. Cyclopropane derivatives. II. The electric moments of some alicyclic compounds. Journal of the American Chemical Society 68: 843–846.
Veeneman, G.H. 2005. Non-steroidal subtype selective estrogens. Current Medicinal Chemistry 12: 1077–1136.
Acknowledgments
This research was supported by the Sookmyung Women’s University Research Grants 2010.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yeo, H.L., Song, Y.S., Ryu, JH. et al. Design, synthesis, and biological evaluation of cyclopropyl analogues of stilbene with raloxifene side chain as subtype-selective ligands for estrogen receptor. Arch. Pharm. Res. 36, 1096–1103 (2013). https://doi.org/10.1007/s12272-013-0134-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-013-0134-2