Abstract
Synthesis, characterization and anticonvulsant properties of new bivalent ligands derived from phenytoin were described. Initial anticonvulsant screening was performed using maximal electroshock (MES) and pentylenetetrazole (PTZ) screens in mice. The neurotoxicity for compounds that showed significant anticonvulsant activity was determined applying the rotorod test. Most of the test compounds were found to be effective in at least one seizure model in a dose of 100 mg/kg. Compound 5e exhibited marked anticonvulsant activity in both MES and PTZ screens. The computer-aided prediction of biological activity was carried out.
Similar content being viewed by others
References
Ahsan, M. J., Samy, J. G., Khalilullah, H., Nomani, M. S., Saraswat, P., Gaur, R., and Singh, A., Molecular properties prediction and synthesis of novel 1,3,4-oxadiazole analogues as potential antimicrobial agents. Bioorg. Med. Chem. Lett., 21, 7246–7250 (2011).
Cramer, J. A., Mintzer, S., Wheless, J., and Mattson, R. H., Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev. Neurother., 10, 885–891 (2010).
Cuadrado, A. and Armijo, J. A., Beneficial interactions between vigabatrin and valproate against seizures induced by pentylenetetrazole in mice. Pharmacol. Res., 51, 489–496 (2005).
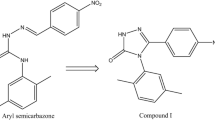
Dimmock, J. R., Pandeya, S. N., Quail, J. W., Allen, T. M., and Kao, G. Y., Evaluation of the semicarbazones, thiosemicarbazones and bis-carbohydrazones of some aryl alicycylic ketones for anticonvulsant and other biological properties. Eur. J. Med. Chem., 30, 303–314 (1995).
Fisher, R. S., Animal models of the epilepsies. Brain Res. Rev., 14, 245–278 (1989).
George, S. R., O’Dowd, B., and Lee, S. P., G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discov., 1, 808–820 (2002).
Greenwood, R. S., Adverse effects of antiepileptic drugs. Epilepsia, 41, 42–52 (2000).
Huber, D., Löber, S., Hübner, H., and Gmeiner, P., Bivalent molecular probes for dopamine D2-like receptors. Bioorg. Med. Chem., 20, 455–466 (2012).
Hudkins, R. L. and DeHaven-Hudkins, D. L., Phenytoin derivatives as potent -ligands. Bioorg. Med. Chem. Lett., 4, 2185–2188 (1994).
Kaminski, K., Rzepka, S., and Obniska, J., Synthesis and anticonvulsant activity of new 1-[2-oxo-2-(4-phenylpiperazin-1-yl)ethyl]pyrrolidine-2,5-diones. Bioorg. Med. Chem. Lett., 21, 5800–5803 (2011).
Kitano, Y., Usui, C., Takasuna, K., Hirohashi, M., and Nomura, M., Increasing-current electroshock seizure test: A new method for assessment of anti- and pro-convulsant activities of drugs in mice. J. Pharmacol. Toxicol. Methods, 35, 25–29 (1996).
Krall, R. L., Penry, J. K., White, B. G., Kupferberg, H. J., and Swinyard, E. A., Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia, 19, 409–428 (1998).
Lenkowski, P. W., Batts, T. W., Smith, M. D., Ko, S. H., Jones, P. J., Taylor, C. H., McCusker, A. K., Davis, G. C., Hartmann, H. A., White, H. S., Brown, M. L., and Patel, M. K., A pharmacophore derived phenytoin analogue with increased affinity for slow inactivated sodium channels exhibits a desired anticonvulsant profile. Neuropharmacology, 52, 1044–1054 (2007).
Lipinski, C. A., Lombardo, F., Dominy, B. W., and Feeney, P. J., Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev., 46, 3–26 (2001).
Löscher, W., Nau, H., Marescaux, C., and Vergnes, M., Comparative evaluation of anticonvulsant and toxic potencies of valproic acid and 2-en-valproic acid in different animal models of epilepsy. Eur. J. Pharmacol., 99, 211–218 (1984).
Löscher, W., Honack, D., Fassbender, C. P., and Nolting, B., The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs, III: Pentylenetetrazole seizure models. Epilepsy Res., 8, 171–189 (1991).
Löscher, W., New visions in the pharmacology of anticonvulsion. Eur. J. Pharmacol., 342, 1–13 (1998).
Löscher, W. and Schmidt, D., New horizons in the development of antiepileptic drugs: Innovative strategies. Epilepsy Res., 69, 183–272 (2006).
Obniska, J., Kaminski, K., Dorota, S., and Pichor, J., Synthesis and anticonvulsant activity of new N-[(4-arylpiperazin-1-yl)-alkyl] derivatives of 3-phenyl-pyrrolidine-2,5-dione. Eur. J. Med. Chem., 44, 2224–2233 (2009).
Ouchi, M., Inoue, Y., Liu, Y., Nagamune, S., Nakamura, S., Wada, K., and Hakushi, T., Convenient and efficient tosylation of oligoethylene glycosl and the related alcohols in tetrahydrofuran-water in the presence of sodium hydroxide. Bull. Chem. Soc. Jpn., 63, 1260–1262 (1990).
Pandeya, S. N., Yogeeswari, P., and Stables, J. P., Synthesis and anticonvulsant activity of 4-bromophenyl substituted aryl semicarbazones. Eur. J. Med. Chem., 35, 879–886 (2000).
Paxton, J. W., Rowell, F. J., and Ratcliffe, J. G., Production and characterisation of antisera to diphenylhydantoin suitable for radioimmunoassay. J. Immunol. Methods, 10, 317–327 (1976).
Poupaert, J. H., Mergen, F., and Lerot, T., Synthesis of bivalent ligands derived from phenytoin. Bull. Soc. Chim. Belg., 97, 469–470 (1988).
Refsgaard, H. H., Jensen, B. F., Brockhoff, P. B., Padkjaer, S. B., Guldbrandt, M., and Christensen, M. S., In silico prediction of membrane permeability from calculated molecular parameters. J. Med. Chem., 48, 805–811 (2005).
Rogawski, M. A., Molecular targets versus models for new antiepileptic drug discovery. Epilepsy Res., 68, 22–28 (2006).
Scheurer, M. L. and Pedley, T. A., The evaluation and treatment of seizures. N. Engl. J. Med., 323, 1468–1474 (1990).
Schlögl, K., Wessely, F., and Korger, G., Zur kenntnis der hydantoinpeptide. Monatsh. Chem., 83, 493–512 (1952).
Upton, N., Mechanisms of action of new antiepileptic drugs: rational design and serendipitous findings. Trends Pharmacol. Sci., 15, 456–463 (1994).
Veber, D. F., Johnson, S. R., Cheng, H. Y., Smith, B. R., Ward, K. W., and Kapple, K. D., Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem., 45, 2615–2623 (2002).
Wright, D. and Usher, L., Multivalent binding in the design of bioactive compounds. Curr. Org. Chem., 5, 1107–1131 (2001).
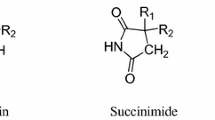
Zha, C., Brown, G. B., and Brouillette, W. J., Synthesis and structure-activity relationship studies for hydantoins and analogues as voltage-gated sodium channel ligands. J. Med. Chem., 47, 6519–6528 (2004).
Zhao, Y. H., Abraham, M. H., Le, J., Hersey, A., Luscombe, C. N., Beck, G., Sherborne, B., and Cooper, I., Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res., 19, 1446–1457 (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Botros, S., Khalil, N.A., Naguib, B.H. et al. Phenytoin-based bivalent ligands: Design, synthesis and anticonvulsant activity. Arch. Pharm. Res. 35, 2105–2116 (2012). https://doi.org/10.1007/s12272-012-1207-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-012-1207-3