Abstract
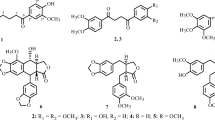
A new phenylpropanoid derivative (1), along with five phenylpropanoids (2–6), two monoepoxy lignans (8–9), one bisepoxy lignan (10), two cyclolignans (11–12), six neolignans (7, 13–17), two mixed lignan-neolignans (18–19), two lignan glycosides (20–21), and four flavonolignans (22–25), were isolated from the stems and twigs of Euonymus acanthocarpus. Compounds 2–3, 6–8, 12, and 14–25 were obtained from Celastraceae family for the first time, and compounds 5 and 9 were isolated from Euonymus genus for the first time. All the compounds were tested for cytotoxicity against SK-OV-3 and MCG-803 human tumor cell lines. Compounds 3, 10, 12, and 18 showed weak cytotoxicity against SK-OV-3 cell line, and compounds 3–4, 10–13, and 19 showed weak cytotoxicity against MCG-803 cell line.
Similar content being viewed by others
References
Barakat, H. H., Nawwar, M. A. M., Buddrus, J., and Linscheid, M., Niloticol, a phenolic glyceride and two phenolic aldehydes from the roots of Tamarix Nilotica. Phytochemistry, 26, 1837–1838 (1987).
Chen, C. Y., Wu, T. Y., Chang, F. R., and Wu, Y. C., Lignans and kauranes from the stems of Annona cherimola. J. Chin. Chem. Soc., 45, 629–634 (1998).
Fang, Z. F. and Hua, H. M., Chemical constituents and bioactivities of plants from the genus euonymus. World Phytomedicines, 22, 6–11 (2007).
Flora of China. Science Press, Beijing, 45, p. 21, (1999).
Greca, M. D., Ferrara, M., Fiorentino, A., Monaco, P., and Previtera, L., Antialgal compounds from Zantedeschia aethiopica. Phytochemistry, 49, 1299–1304 (1998).
Hitoshi, T., Masaru, H., Kazuhiko, I., and Kazuo, I., Total synthesis of silychristin, an antihepatotoxic flavonolignan. Chem. Pharm. Bull., 37, 1441–1145 (1989).
Hong, S. S., Han, X. H., Hwang, J. S., Lee, K. S., Lee, M. K., Ro, J. S., and Hwang, B. Y., Lignans from the stem barks of Kalopanax septemlobus. Nat. Prod. Sci., 12, 201–204 (2006).
Hsiao, J. J. and Chiang, H. C., Lignans from the wood of Aralia Bipinnat. Phytochemistry, 39, 899–902 (1995).
Keiji, M., Takashi, T., Takashi, S., and Akira, S., Lignans of Larix Leptolepis. Phytochemistry, 19, 449–453 (1980).
Kim, N. C., Grag, T. N., Sparacino, C. M., Wani, M. C., and Wall, M. E., Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org. Biomol. Chem., 1, 1684–1689 (2003).
Kuo, H. T., Peng, C. F., Huang, H. Y., Lin, C. H., Chen, I. S., and Tsai, I. L., Chemical constituents and antitubercular activity of Formosan Pisonia umbellifera. Planta Med., 77, 736–741 (2011).
Lee, D. Y. and Liu, Y., Molecular structure and stereochemistry of silybin A, silybin B, isosilybin A, and isosilybin B, isolated from Silybum marianum (milk thistle). J. Nat. Prod., 66, 1171–1174 (2003).
Lee, M. K., Sung, S. H., Lee, H. S., Cho, J. H., and Kim, Y. C., Lignan and neolignan glycosides from Ulmus davidiana var. japonica. Arch. Pharm. Res., 24, 198–201 (2001).
Li, L. and Seeram, N. P., Further investigation into maple syrup yields 3 new lignans, a new phenylpropanoid, and 26 other phytochemicals. J. Agric. Food Chem., 59, 7708–7716 (2011).
Lourith, N., Katayama, T., Ishikawa, K., Suzuki, T., Biosynthesis of a syringyl 8-O-4′ neolignan in Eucommia ulmoides: formation of syringylglycerol-8-O-4′-(sinapyl alcohol) ether from sinapyl alcohol. J. Wood Sci., 51, 379–386 (2005).
Ma, C., Zhang, H. J., Tan, G. T., Hung, N. V., Cuong, N. M., Soejarto, D. D., and Fong, H. H., Antimalarial compounds from Grewia bilamellata. J. Nat. Prod., 69, 346–350 (2006).
Ma, J., Dey, M., Yang, H., Poulev, A., Pouleva, R., Dorn, R., Lipsky, P. E., Kennelly, E. J., and Raskin, I., Anti-inammatory and immunosuppressive compounds from Tripterygium wilfordii. Phytochemistry, 68, 1172–1178 (2007).
Matsuda, N. and Kikuchi, M., Studies on the constituents of lonicera species X. neolignan glycosides from the leavesof Lonicera gracilipes var. glandulosa maxim. Chem. Pharm. Bull., 44, 1676–1679 (1996).
Medicinal Herb of Guizhou. People Press of Guizhou, Guizhou, pp. 599–600, (1970).
Nagafuji, S., Okabe, H., Akahane, H., and Abe, F., Trypanocidal constituents in plants 4. Withanolides from the aerial parts of Physalis angulata. Biol. Pharm. Bull., 27, 193–197 (2004).
Sadhu, S. K., Phattanawasin, P., Choudhuri, M. S. K., Ohtsuki, T., and Ishibashi, M., A new lignan from Aphanamixis polystachya. J. Nat. Med., 60, 258–260 (2006).
Takashi, I., Stephen, V. J. B., John, C., Alex, D., and Mitsuhiro, T., The absolute configuration of the four stereoisomers of trans-anethole diol (1′-(4″-Methoxyphenyl)-1,2-propanediol), a metabolite of anethole in the rat. Tetrahedron, 7, 3113–3118 (1996).
Tominaga, H., Ishiyama, M., Ohseto, F., Sasamoto, K., Hamamoto, T., Suzuki, K., and Watanabe, M., A watersoluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun., 36, 47–50 (1999).
Xiong, L., Zhu, C. G., Li, Y. R., Tian, Y., Lin, S., Yuan, S. P., Hu, J. F., Hou, Q., Chen, N. H., Yang, Y. C., and Shi, J. G., Lignans and neolignans from Sinocalamus affinis and their absolute configurations. J. Nat. Prod., 74, 1188–1120 (2011).
Xu, Z. R., Chai, X. Y., Bai, C. C., Ren, H. Y., Lu, Y. N., Shi, H. M., and Tu, P. F., Xylocosides A-G, phenolic glucosides from the stems of Xylosma controversum. Helv. Chim. Acta, 91, 1346–1354 (2008).
Yang, C. J., Tang, W. Z., Wang, X. J., and Zhang, Z. H., Chemical constituents from the fruits of Ailanthus altissima (Mill.) Swingle. Chinese Traditional Patent Medicine, 32, 1176–1179 (2010).
Yang, J. Q., He, W. J., Tan, N. H., Chu, H. B., Zhang, Y. M., Mei, W. L., and Dai, H. F., Chemical constituents of Pedicularis cephalantha franch and P. siphonantha Don. Nat. Prod. Res. Dev., 21, 600–603 (2009).
Yang, Y., Jiang, J. Z., Qimei, L. B., Yan, X. J., Zhao, J. X., Yuan, H. Z., Qin, Z. H., and Wang, M. G., The fungicidal terpenoids and essential oil from Litsea cubeba in Tibet. Molecules, 15, 7075–7082 (2010).
Zhang, Z. Z., Guo, D. A., Li, C. L., Zheng, J. H., Kazuo, K., Jia, Z. H., and Tamotsu, N., Studies on the lignan glycosides from Gaultheria Yunnanensis. Acta Pharm. Aceu. Tica. Sinica, 34, 49–52 (1999).
Zhou, D. Z., Yi, Y. H., Mao, S. L., Lu, T. S., Tang, H. F., Zou, Z. R., and Zhang, S. Y., The lignins from Torreya grandis cv. Merrilli. Yao Xue Xue Bao, 39, 269–271 (2004).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, J.X., Ren, J., Qin, J.J. et al. Phenylpropanoids and lignanoids from Euonymus acanthocarpus . Arch. Pharm. Res. 35, 1739–1747 (2012). https://doi.org/10.1007/s12272-012-1005-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-012-1005-y