Abstract
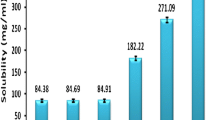
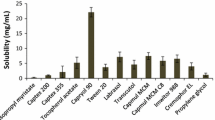
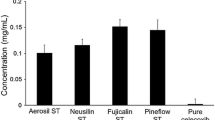
The main purpose of this study was to evaluate the effect of a mixed drug solution containing a surfactant and β-cyclodextrin (β-CD) on the solubility and bioavailability of a poorly watersoluble drug, flurbiprofen. Solubility, dissolution and in vivo pharmacokinetics of flurbiprofen in the presence of surfactant, β-CD or mixture of surfactant and β-CD were investigated. Among the surfactants tested, Tween 80 produced the highest improvement in the aqueous solubility of flurbiprofen. The solubility of flurbiprofen increased linearly as a function of β-CD, resulting in B8 type that suggested a formation of inclusion complex in a molar ratio of 1:1. The solubility of flurbiprofen increased further when Tween 80 was included in addition to β-CD, suggesting that a micelle formation in the presence of Tween 80 was the likely reason for additional increase. Furthermore, the data suggested that Tween 80 did not interfere with the inclusion interaction between flurbiprofen and β-CD. The solubility of flurbiprofen was the highest in the mixed system containing 1.3 mM β-CD and 0.3% w/v Tween 80, and the maximum solubility of 160 μg/mL was achieved. Consistent with the enhanced solubility, the plasma exposure (both AUC and Cmax) of flurbiprofen when dosed as the mixed system was significantly higher (as much as 2 to 3-fold) than that without surfactant or β-CD, with surfactant alone, or with β-CD alone. Therefore, the mixed system consists of surfactant and β-CD could be used as an effective oral dosage form to improve bioavailability of poorly water soluble drugs such as flurbiprofen.
Similar content being viewed by others
References
Anderson, B. D. and Conradi, R. A., Predictive relationships in the water solubility of salts of a nonsteroidal antiinflammatory drug. J. Pharm. Sci., 74, 815–820 (1985).
Barone, J. A., Moskovitz, B. L., Guarnieri, J., Hassell, A. E., Colaizzi, J. L., Bierman, R. H., and Jessen, L., Enhanced bioavailability of itraconazole in hydroxypropyl β-cyclodextrin solution versus capsules in healthy volunteers. Antimicrob. Agents Chemother., 42, 1862–1865 (1998).
Carrier, R. L., Miller, L. A., and Ahmed, I., The utility of cyclodextrins for enhancing oral bioavailability. J. Control. Release, 123, 78–99 (2007).
Charman, W. N. and Stella, V. J., Transport of lipophilic molecules by the intestinal lymphatic system. Adv. Drug Deliv. Rev., 7, 1–14 (1991).
Choi, H. G., Kim, D. D., Jun, H. W., Yoo, B. K., and Yong, C. S., Improvement of dissolution and bioavailability of nitrendipine by inclusion in hydroxypropyl-beta-cyclodextrin. Drug Dev. Ind. Pharm., 29, 1085–1094 (2003).
Choi, H. G., Lee, B. J., Han, J. H., Lee, M. K., Park, K. M., Yong, C. S., Rhee, J. D., Kim, Y. B., and Kim, C. K., Terfenadine-beta-Cyclodextrin inclusion complex with antihistaminic activity enhancement. Drug Dev. Ind. Pharm., 27, 857–862 (2001).
Davies, N. M., Clinical pharmacokinetics of flurbiprofen and its enantiomers. Clin. Pharmacokinet., 28, 100–114 (1995).
Edwards, D. A., Luthy, R. G., and Liu, Z., Solubilization of polycyclic aromatic hydrocarbons in micellar nonionic surfactant solutions. Environ. Sci. Technol., 25, 127–133 (1991).
Gershanik, T. and Benita, S., Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur. J. Pharm. Biopharm., 50, 179–188 (2000).
Habib, M. J., Phan, M. T., and Owusu-Ababio, G., Dissolution profiles of flurbiprofen in phospholipid solid dispersions. Drug Dev. Ind. Pharm., 24, 1077–1082 (1998).
Higuchi, T. and Connors, K. A., Phase-solubility techniques. Adv. Anal. Chem. Instr., 4, 117–212 (1965).
Horter, D. and Dressman, J. B., Influence of physicochemical properties on dissolution of drugs in the gastrointestinal tract. Adv. Drug Deliv. Rev., 46, 75–87 (2001).
Kim, C. K., Yoon, Y. S., and Kong, J. P., Preparation and evaluation of flurbiprofen dry elixir as a novel dosage form using a spray-drying technique. J.Y., Int. J. Pharm., 120, 21–31 (1995).
Lee, E. J., Lee, S. W., Choi, H. G., and Kim, C. K., Bioavailability of cyclosporin A dispersed in sodium lauryl sulfate-dextrin based solid microspheres. Int. J. Pharm., 218, 125–131 (2001).
Li, D. X., Oh, Y. K., Lim, S. J., Kim, J. O, Yang, H. J., Sung, J. H., Yong, C. S., and Choi, H. G., Novel gelatin microcapsule with bioavailability enhancement of ibuprofen using spray drying technique. Int. J. Pharm., 355, 277–284 (2008).
Li, P., Ghosha, A., Wagnera, R. F., Krillb, S., Joshia, Y. M., and Serajuddin, A. T. M., Effect of combined use of nonionic surfactant on formation of oil-in-water microemulsions. Int. J. Pharm., 288, 27–34 (2005).
Muraoka, A., Tokumura, T., and Machida, Y., Evaluation of the bioavailability of flurbiprofen and its β-cyclodextrin inclusion complex in four different doses upon oral administration to rats. Eur. J. Pharm. Biopharm., 58, 667–671 (2004).
Newa, M., Bhandari, K. H., Li, D. X., Kim, J. O., Yoo, D. S., Kim, J. A., Yoo, B. K., Woo, J. S., Lyoo, W. S., Yong, C. S., and Choi, H. G., Preparation and evaluation of immediate release ibuprofen solid dispersions using polyethylene glycol 4000. Biol. Pharm. Bull., 31, 939–945 (2008).
Ono, N., Hirayama, F., Arima, H., and Uekama, K., Analysis of the phase solubility diagram of a phenacetin/competitor/β-cyclodextrin ternary system, involving competitive inclusion complexation. Chem. Pharm. Bull., 49, 78–81 (2001).
Park, K. M. and Kim, C. K., Preparation and evaluation of flurbiprofen-loaded microemulsion for parenteral delivery. Int. J. Pharm., 30, 173–179 (1999).
Park, K. M., Lee, M. K., Hwang, K. J., and Kim, C. K., Phospholipid-based microemulsions of flurbiprofen by the spontaneous emulsification process. Int. J. Pharm., 183, 145–154 (1999).
Prabagar, B., Yoo, B. K., Woo, J. S., Kim, J. A., Rhee, J. D., Piao, M. G., Choi, H. G., and Yong, C. S., Enhanced Bioavailability of Poorly Water-Soluble Clotrimazole by Inclusion with β-Cyclodextrin. Arch. Pharm. Res., 30, 249–254 (2007).
Society of Toxicology (SOT), Guilding Priciples in the Use of Animals in Toxicology, (1999). www.toxicology.org/AI/FA/guidingprinciples.pdf.
Volkering, F., Breure, A. M., Van Andel, J. G., and Rulkens, W. H., Influence of nonionic surfactants on bioavailability and biodegradation of polycyclic aromatic hydrocarbons. Environ. Microbiol., 61, 1699–1705 (1995).
Waleczeka, K. J., Cabral Marques, H. M., Hempelc, B., and Schmidt, P. C., Phase solubility studies of pure (−)-alphabisabolol and camomile essential oil with beta-cyclodextrin. Eur. J. Pharm. Biopharm., 55, 247–251 (2003).
Yong, C. S., Yang, C. H., Rhee, J. D., Lee, B. J., Kim, D. C., Kim, D. D., Kim, C. K., Choi, J. S., and Choi, H. G., Enhanced rectal bioavailability of ibuprofen in rats by poloxamer 188 and menthol. Int. J. Pharm., 269, 169–176 (2004).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, D.X., Han, M.J., Balakrishnan, P. et al. Enhanced oral bioavailability of flurbiprofen by combined use of micelle solution and inclusion compound. Arch. Pharm. Res. 33, 95–101 (2010). https://doi.org/10.1007/s12272-010-2231-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-010-2231-9