Abstract
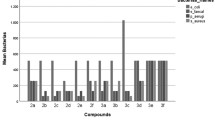
New 4-thiazolidinone derivatives of benzilic acid (α,α-diphenyl-α-hydroxyacetic acid) have been synthesized and evaluated for antibacterial and antifungal activities. The reaction of 1- (α,α-diphenyl-α-hydroxy)acetyl-4-alkyl/arylthiosemicarbazides with ethyl 2-bromopropionate gave 3-alkyl/aryl-2-[((α,α-diphenyl-α-hydroxy)acetyl)hydrazono]-5-methyl-4-thiazolidinone derivatives. Their antibacterial and antifungal activities were evaluated against S. aureus ATCC 29213, P. aeruginosa ATCC 27853, E. coli ATCC 25922, C. albicans ATCC 10231, C. parapsilosis ATCC 22019, C. krusei ATCC 6258, T. mentagrophytes var. erinacei NCPF 375, M. gypseum NCPF 580 and T. tonsurans NCPF 245. 3e, 3f, 3g and 3h showed the highest antibacterial activity. Particularly 3a and 3e showed the highest antifungal activities against C. parapsilosis ATCC 22019, T. tonsurans NCPF 245 and M. gypseum NCPF 580.
Similar content being viewed by others
References
Ali, M. M. and Hassan, S. A., Role of some newly synthesized tetrahydronaphthalenthiazol derivatives as anticancer compounds. Int. J. Cancer Res., 3, 103–110 (2007).
Amin, K. M., Abdel Rahman, D. E., and Al-Eryani, Y. A., Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem., 16, 5377–5388 (2008).
Aquino, T. M., Liesen, A. P., Silva, R. E. A., Lima, V. T., Carvalho, C. S., Faria, A. R., Araújo, J. M., Lima, J. G., Alves, A. J., Melo, E. J. T., and Góes, A. J. S., Synthesis, anti-Toxoplasma gondii and antimicrobial activities of benzaldehyde 4-phenyl-3-thiosemicarbazones and 2-[(phenylmethylene)hydrazono]-4-oxo-3-phenyl-5-thiazolidineacetic acids. Bioorg. Med. Chem., 16, 446–456 (2008).
Archana, V. K. and Srivastava, A. K., Synthesis of newer thiadiazolyl and thiazolidinonyl quinazolin-4(3H)-ones as potential anticonvulsant agents. Eur. J. Med. Chem., 37, 873–882 (2002).
Balzarini, J., Orzeszko, B., Maurin, J. K., and Orzeszko, A., Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem., 42, 993–1003 (2007).
Bhati, S. K. and Kumar, A., Synthesis of new substituted azetidinoyl and thiazolidinoyl-1,3,4-thiadiazino[6,5-b]indoles as promising anti-inflammatory agents. Eur. J. Med. Chem., 43, 2323–2330 (2008).
Bondock, S., Khalifa, W., and Fadda, A. A., Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur. J. Med. Chem., 42, 948–954 (2007).
Cesur, N., Cesur, Z., Ergenç, N., Uzun, M., Kiraz, M., Kasimoglu Ö., and Kaya D., Synthesis and antifungal activity of some 2-aryl-3-substituted 4-thiazolidinones. Synthese und antimykotische Aktivität einiger 2-Aryl-3-substituierter 4-Thiazolidinone. Arch. Pharm. (Weinheim), 327, 271–272 (1994).
Çapan, G., Ulusoy, N., Ergenç, N., Ekinci, A. C., and Vidin, A., Synthesis and anticonvulsant activity of new 3-[(2-furyl)carbonyl]amino-4-thiazolidinone and 2-[(2-furyl) carbonyl]hydrazono-4-thiazoline derivatives. Il Farmaco, 51, 729–732 (1996).
Çapan, G., Ulusoy, N., Ergenç, N., and Kiraz, M., New 6-phenylimidazo[2,1-b]thiazole derivatives: Synthesis and antifungal activity. Monatsh. Chem., 130, 1399–1407 (1999).
De Lima, W.T., De Lima, J. G., and Góes, A.J. S., Spectros. Lett., 35, 137–144 (2002).
Diurno, M. V., Mazzoni, O., Correale, G., Monterrey, I. G., Calignano, A., La Rana, G., and Bolognese, A., Synthesis and structure-activity relationships of 2-(substituted phenyl)-3-[3-(N,N-dimethylamino)propyl]-1,3-thiazolidin-4-ones acting as H1-histamine antagonists. Il Farmaco, 54, 579–583 (1999).
Ergenç, N., Ilhan, E., and Ötük, G., Synthese und biologische Wirkung einiger 1,4-disubstituierter Thiosemicarbazide und deren 1,2,4-Triazol-5-thion-Derivate. Pharmazie, 47, 59–60 (1992).
Farghaly, A. M., Habib, N. S., Khalil, M. A., El-Sayed, O. A., and Bistawroos, A. E., Synthesis of novel 2-substituted quinoline derivatives: Antimicrobial, inotropic, and chronotropic activities. Arch. Pharm. (Weinheim), 323, 247–251 (1990).
Fernandez-Torres, B., Cabanes, F. J., Carillo-Munoz, A., Esteban, A., Inza, I., Abarca, L., and Guarro, J., Collaborative evaluation of optimal antifungal susceptibility testing canditions for dermatophytes. J. Clin. Microbiol., 40, 3999–4003 (2002).
Frère, J.-M., Beta-lactamases and bacterial resistance to antibiotics. Mol. Microbiol., 16, 385–395 (1995).
Goel, B., Ram, T., Tyagi, R., Bansal, E., Kumar, A., Mukherjee, D., and Sinha, J. N., 2-Substituted-3-(4-bromo-2-carboxyphenyl)-5-methyl-4-thiazolidinones as potential anti-inflammatory agents. Eur. J. Med. Chem., 34, 265–269 (1999).
Gürsoy, A. and Terzioglu, N., Synthesis and isolation of new regioisomeric 4-thiazolidinones and their anticonvulsant activity. Turk. J. Chem., 29, 247–254 (2005).
Ilhan, E. and Ergenç, N., Synthese und Reaktivität von 3-substituierten Thiazolidin-2,4-dion-2-(α, α-diphenyl-α-hydroxyacetyl)hydrazonen 3-Substituted Thiazolidine-2,4-dione-2-(α,α-diphenyl-α-hydroxyacetyl)hydrazones. Arch. Pharm., 325, 453–454 (1992).
Ilhan, E., Ergenç, N., Ulusoy, N., and Ötük-Sanis, G., Synthese und antimikrobie einiger 4-Arylidenamino-3-(α,α-diphenyl-α-hydroxymethyl)-1,4-dihydro-5H-1,2,4-triazol-5-thione und 6-Aryl-3-(α,α-diphenyl-α-hydroxymethyl)-7H-s-triazolo[3,4-b][1,3,4]thiadiazine. Pharmazie, 51, 123–124 (1996).
Ilhan, E., Ergenç, N., Uzun, M., and Kaya, D., Synthese von 6-Benzyliden-2-(α,α-diphenyl-α-hydroxyacetyl)-thiazolo [3,2-b]-s-triazol-5-onen als potentiell biologisch wirksame Stoffe. Arch. Pharm., 327, 825–826 (1994).
Kaplancikli, Z. A., Turan-Zitouni, G., Özdemir, A., and Revial, G., Synthesis and anticandidal activity of some imidazopyridine derivatives. J. Enzyme Inhib. Med. Chem., 23, 866–870 (2008).
Karali, N., Gürsoy, A., Kandemirli, F., Shvets, N., Kaynak, F. B., Özbey, S., Kovalishyn, V., and Dimoglo, A., Synthesis and structure-antituberculosis activity relationship of 1H-indole-2,3-dione derivatives. Bioorg. Med. Chem., 15, 5888–5904 (2007).
Kavitha, C. V., Basappa, S., Swamy, N., Mantelingu, K., Doreswamy, S., Sridhar, M. A., Prasad, S., and Rangappa, K. S., Synthesis of new bioactive venlafaxine analogs: Novel thiazolidin-4-ones as antimicrobials. Bioorg. Med. Chem., 14, 2290–2299 (2006).
Kumar, A. and Rajput, C. S., Synthesis and anti-inflammatory activity of newer quinazolin-4-one derivatives. Eur. J. Med. Chem., 44, 83–90 (2009).
Kumar, P. R., Yadav, M. S., Kumar, M. M. K., and Rao, T. S., Synthesis and antimicrobial activity of some new substituted aryloxy-4-thiazolidinones. E-J. Chem., 3, 44–48 (2006).
Küçükgüzel, G., Kocatepe, A., De Clercq, E., Sahin, F., and Güllüce, M., Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem., 41, 353–359 (2006).
Küçükgüzel, S. G., Oruç, E. E., Rollas, S., Sahin, F., and Özbek, A., Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur. J. Med. Chem., 37, 197–206 (2002).
Mishra, P., Lukose, T., and Kashaw, S. K., Synthesis and antimicrobial evaluation of some novel 2-imino-3-(4’-carboxamido pyridyl)-5-arylidene-4-thiazolidinones and their brominated derivatives. Indian J. Pharm. Sci., 69, 665–668 (2007).
National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing filamentous fungi; Approved standard. M38-A. National Commitee for Clinical Laboratory Standards (2002).
Özkirimli, S., Kazan, F., and Tunali, Y., Synthesis, antibacterial and antifungal activities of 3-(1,2,4-triazol-3-yl)-4-thiazolidinones. J. Enzymn Inhib. Med. Chem., 23, 1–6 (2008).
Rao, A., Balzarini, J., Carbone, A., Chimirri, A., De Clercq, E., Monforte, A. M., Monforte, P., and Pannecouque, C., Zappalà M., 2-(2,6-Dihalophenyl)-3-(pyrimidin-2-yl)-1,3-thiazolidin-4-ones as non-nucleoside HIV-1 reverse transcriptase inhibitors. Antiviral Res., 63, 79–84 (2004).
Rao, A., Balzarini, J., Carbone, A., Chimirri, A., De Clercq, E., Monforte, A. M., Monforte, P., Pannecouque, C., and Zappalà, M., Synthesis of new 2,3-diaryl-1,3-thiazolidin-4-ones as anti- HIV agents. Il Farmaco, 59, 33–39 (2004).
Rao, A., Carbone, A., Chimirri, A., De Clercq, E., Monforte, A. M., Monforte, P., Pannecouque, C., and Zappalà, M., Synthesis and anti-HIV activity of 2,3-diaryl-1,3-thiazolidin-4-ones. Il Farmaco, 58, 115–120 (2003).
Rawal, R. K., Tripathi, R., Katti, S. B., Pannecouque, C., and De Clercq, E., Design and synthesis of 2-(2,6-dibromophenyl)-3-heteroaryl-1,3-thiazolidin-4-ones as anti-HIV agents. Eur. J. Med. Chem., 43, 2800–2806 (2008).
Rida, S. M., Ashour, F. A., El-Hawash, S. A. M., ElSemary, M. M., Badr, M. H., and Shalaby, M. A., Synthesis of some novel benzoxazole derivatives as anticancer, anti-HIV-1 and antimicrobial agents. Eur. J. Med. Chem., 40, 949–959 (2005).
Shah, T. J. and Desai, V. A., Synthesis of some novel fluorinated 4-thiazolidinones containing amide linkages and their antimicrobial screening. Arkivoc, 14, 218–228 (2007).
Siddiqui, I. R., Singh, P. K., Singh, J., and Singh, J., Synthesis and fungicidal activity of novel 4,4’-bis(2″-aryl-5″-methyl/unsubstituted-4″-oxo-thiazolidin-3″-yl)bibenzyl. J. Agric. Food Chem., 51, 7062–7065 (2003).
Ulusoy, N., çapan, G., Ergenç, N., Ekinci, A. C., and Vidin, A., Synthesis, characterization and anticonvulsant activity of new 4-thiazolidinone and 1,2,4-triazole-3-thione derivatives. Acta Pharm. Turc., 40, 5–8 (1998).
Ulusoy, N., çapan, G., Ergenç, N., Ötük, Sanis, G., Kiraz, M., and Kaya, D., Synthesis and antimicrobial activity of novel imidazo[2,1-b]thiazolyl acetyl amino/hydrazono 4-thiazolidinones. Synthesis and antimicrobial activity of novel imidazo[2,1-b]thiazolyl acetyl amino/hydrazono 4-thiazolidinones. Acta Pharm. Turc., 39, 181–186 (1997).
Ulusoy, N., Ergenç, N., Ekinci, A. C., and Özer, H., Synthesis and anticonvulsant activity of some new arylidenehydrazides and 4-thiazolidinones. Monatsh. Chem., 127, 1197–1202 (1996).
Ulusoy, N., Kiraz, M., and Küçükbasmaci, Ö., New 6-(4-Bromophenyl)-imidazo[2,1-b]thiazole derivatives: Synthesis and antimicrobial activity. Monatsh. Chem., 133, 1305–1315 (2002).
Vigorita, M. G., Ottanà, R., Monforte, F., Maccari, R., Trovato, A., Monforte, M. T., Taviano, M. F., Synthesis and antiinflammatory, analgesic activity of 3,3’-(1,2-ethanediyl)-bis[2-aryl-4-thiazolidinone] chiral compounds. Part 10. Bioorg. Med. Chem. Lett., 11, 2791–2794 (2001).
Wayne, P. A., Clinical and Laboratory Standards Institute. Performance standards for antimicrobial testing, 15th informational supplement. M100–S15, (2005).
Wayne, P. A., National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts; Approved standard-2nd Edition. M27–A2, (2002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Güzeldemirci, N.U., Ilhan, E., Küçükbasmaci, Ö. et al. Synthesis and antimicrobial evaluation of new 3-alkyl/aryl-2-[((α,α-diphenyl-α-hydroxy)acetyl)hydrazono]-5-methyl-4-thiazolidinones. Arch. Pharm. Res. 33, 17–24 (2010). https://doi.org/10.1007/s12272-010-2221-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-010-2221-y